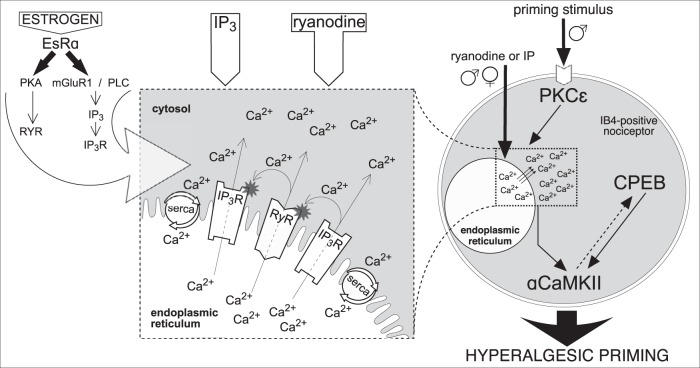
Figure 7.
Schematic diagram of proposed mechanism of reciprocal interaction between IP3 and ryanodine receptors in the induction of hyperalgesic priming. Activation of PKCε (in males, only) by the priming stimulus in the peripheral terminal of an IB4+ nociceptor (right side of figure) triggers a cascade of events that ultimately produces hyperalgesic priming (Aley et al., 2000; Joseph et al., 2003; Joseph and Levine, 2010; Ferrari et al., 2013b), which includes calcium release from ER and requires both ryanodine (RyR) and IP3 (IP3R) receptors, activation of αCaMKII, and cytoplasmic polyadenylation element binding protein (CPEB) (Ferrari et al., 2013b). Direct activation of RyR and IP3R also produces priming, in males and females. We hypothesize that release of calcium ions (Ca2+) from ER stores, causing a local increase in [Ca2+]i (dashed and magnified boxes, middle) (Ehrlich et al., 1994; MacKrill, 1999, 2012) is a key factor required for the induction of priming. The suggested interaction between RyR and IP3R, by Ca2+ released through these ionotropic receptors, is shown in the magnified box. Once outside the ER, Ca2+ can activate nearby RyR and IP3R, increasing [Ca2+]i even more, via CICR, to reach a level high enough to trigger mechanisms, such as calmodulin and αCaMKII (Shakiryanova et al., 2007, 2011; Wong et al., 2009), leading to induction of priming (Ferrari et al., 2013b). The ER calcium pump, SERCA, transports the Ca2+ released into the cytosol, back to stores, to refill them, restricting Ca2+ signals spatially and temporally, a “loop” mechanism that has been associated with the development of neuroplasticity (Pal et al., 2001; Scullin and Partridge, 2010; Kato et al., 2012; Komin et al., 2015; Yasuda and Mukai, 2015). Additional Ca2+ buffering in the cytosol limits the fast diffusion of Ca2+, supporting its local accumulation. Thus, the whole system of RyR and IP3R can be triggered by administration of IP3 or ryanodine. When the concentration of agonists reaches a certain level, the effective doses able to induce priming (Ferrari et al., 2016) (Fig. 2a), the local cascade of propagating CICR becomes self-supported. We believe this “small” (in the scale of the whole cell), but long-lasting, change in Ca2+ homeostasis is a crucial factor for the development of hyperalgesic priming. In contrast, when the Ca2+ signal produced is strong but of short duration, for example, that produced by calcium ionophore, due to the bell-shaped sensitivity of RyR and IP3R for [Ca2+]i, the production of the self-supported long-lasting local [Ca2+]i increase is prevented, and hyperalgesic priming does not develop. Although the direct interaction between the receptors is important to generate amplification, we consider Ca2+ a key mediator of the reported reciprocal modulation of RyR and IP3R, and the amount of Ca2+ initially released is the most important factor in the mechanism, regardless of which receptor is stimulated. This constitutes our paradigm of “the common (shared) amplifier,” postulating the amplification of Ca2+ signals produced by either input (RyR or IP3R), which explains the similarity in the difference between males and females in the sensitivity to be primed by either IP3 or ryanodine. Although this amplification system is present in both males and females, it is less potent in males (a 100,000-fold difference). In regard of the higher sensitivity of females to be primed by ryanodine or IP3, the action of estrogen on EsRα (left) potentiates the amplification system, likely by sensitizing the ER receptors, as suggested by previous reports: modulation of RyR by estrogen-PKA signaling (Lin et al., 2007) or potentiation of IP3R by estrogen through metabotropic glutamate receptor 1, phospholipase C, and generation of IP3 (Tabatadze et al., 2015) (left, large arrow). This latter pathway could also result in tonic sensitization of IP3R in females, due to an increased basal IP3 levels. As shown in Figures 4 and 6, tonic activation of EsR in cultured male nociceptors, by incubation in the presence of β-estradiol, also potentiates their sensitivity to both ryanodine and IP3. This finding suggests that the sensitizing machinery of EsR is also present in males, but probably not active enough due to a lower level (or lack) of estrogen. Nevertheless, there is still a possibility that the EsRα-dependent sensitization in females (as well as in males, when activated) is indirect, due to the suppression of some blocking pathway active in males and females when EsRα is not stimulated. Although little is known about endogenous competitive antagonists shared by RyR and IP3R, according to our “shared amplification system paradigm,” suppression of either RyR and IP3R by their antagonists, including endogenous (Lenzen and Rustenbeck, 1991; Uehara et al., 1996; Vervliet et al., 2015), could indeed reduce the sensitivity of the whole system (as shown in Figs. 1, 2b). Whether this mechanism is involved in the observed difference between males and females in our study remains to be a determined.