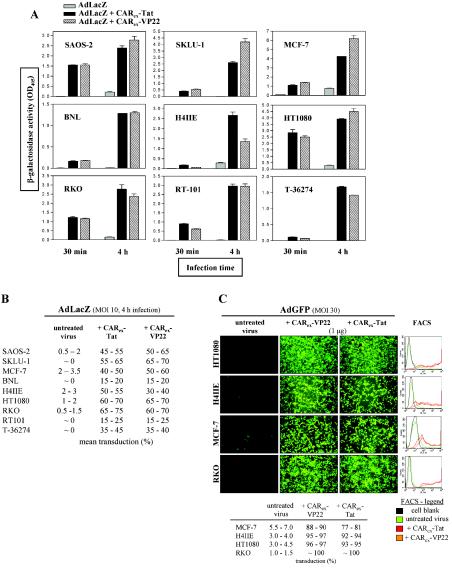
FIG. 3.
Determination of CARex-VP22- and CARex-Tat-mediated adenoviral infection in nonpermissive or partially permissive tumor cell lines. (A) Different target cell types were overlaid with medium containing purified CARex-VP22 or CARex-Tat at a concentration of 2 nM. AdLacZ (MOI, 10) was then added, and infection was carried out for 30 min or 4 h as indicated. After 48 h of incubation, infection efficacy was determined by measuring β-galactosidase activity in extracts from infected cells. (B) The total degree of infection was determined by X-Gal staining. (C) A total of 1 μg of recombinant protein was dissolved in medium and combined with AdGFP (MOI, 30) and incubated for 10 min prior to a 4-h infection of target cells. After 48 h, GFP expression was monitored by fluorescence microscopy, and infection efficacy was determined by FACS analysis. The results indicate that adenovirus treatment with CARex-VP22 and CARex-Tat facilitates effective infection of nonpermissive cells.