Abstract
Background
Five randomized controlled trials recently appeared in the literature demonstrating that early mechanical thrombectomy in patients with acute ischemic stroke is significantly related to an improved outcome. Stent retrievers are accepted as the most effective devices for intracranial thrombectomy.
Objective
To analyze the mechanical properties of stent retrievers, their behavior during retrieval, and interaction with different clots and to identify device features that might correlate with the effectiveness of thrombus removal.
Materials and methods
All stent retrievers available in France up to June 2015 were evaluated by mechanical and functional tests aimed at investigating the variation of their radial force and their behavior during retrieval. Devices were also tested during in vitro thrombectomies using white and red experimental thrombi produced with human blood. Functional tests and in vitro thrombectomies were conducted using a rigid 3D printed vascular model.
Results
Mechanical tests showed a variation in radial force during retrieval for each stent. A constant radial force during retrieval was related to continuous cohesion over the vessel wall and a higher rate of clot removal efficacy. All stent retrievers failed when interacting with white large thrombi (diameter ≥6 mm).
Conclusions
None of the tested devices were effective in removing white clots of large diameter (≥6 mm). Constant radial force during retrieval allows constant cohesion to the vessel wall and pressure over the clot; such features allow for a higher rate of clot removal.
Keywords: Stroke, Thrombectomy, Stent
Introduction
The results of five randomized controlled trials recently published showed that early mechanical thrombectomy in patients presenting with acute ischemic stroke due to a large vessel occlusion is related to improved functional outcome and reduced mortality as compared with standalone intravenous fibrinolysis (IVF).1–5 Stent retrievers (STRs) were used mainly in these trials and are nowadays recognized as the most effective devices for intracranial thrombectomy. The STR is delivered via a femoral artery approach through a microcatheter and deployed within the occluding thrombus. Subsequently, the device and the microcatheter are gently recovered as a single unit from the patient's body. The interaction between device and thrombus is influenced by multiple factors: device mechanical characteristics, device behavior during retrieval, and thrombus biomechanics and consistencies.
In this study we experimentally evaluated the interaction between STRs and different types of thrombi. Furthermore, we analyzed mechanical properties of various devices in order to investigate their interaction with the clot and their behavior during retrieval. The aim of this study was to try to identify any properties of the mechanical device that correlate with a higher rate of thrombus removal.
Material and methods
STRs analyzed in this study are listed in table 1. Each device was evaluated by mechanical and functional tests.
Table 1.
List of thrombectomy devices evaluated in this study
| Device | Size* |
|---|---|
| Trevo Provue (Stryker, Kalamazoo, Michigan, USA)† | 4–20/3–20 |
| Catch (Balt, Montmorency, France)‡ | 3–15/4–20/6–30 |
| Eric (Microvention, Aliso Viejo, California, USA)† | 3–20/4–24/6–44 |
| Preset (Phenox, Bochum, Germany)‡ | 4–20/6–30 |
| Preset LT (Phenox, Bochum, Germany)‡ | 3–20/4–20 |
| Embotrap (Nauravi, Galway, Ireland)† | 5–21 |
| Separator 3D (Penumbra Inc, Alameda, California, USA)† | 4.5–26 |
| Revive (Codman, Raynham, Massachusetts, USA)† | 4.5–22 |
| Mindframe (Medtronic, Irvine, California, USA)† | 3–23 |
| Solitaire FR (Medtronic, Irvine, California, USA)‡ | 4–20/6–30 |
*For each device, the first value refers to the nominal diameter, the second value refers to the length expressed in mm.
†Complete axial section device.
‡Incomplete axial section device.
Mechanical tests
Mechanical tests aimed to measure the radial force exerted by the stent in two specific conditions: upon deployment and during the retrieval.
Flat plate compression test
This test measured the STR outward radial force density6; it corresponds to the force exerted by the device against a flat surface during compression. Tests were performed at room temperature with a tensile test machine as follows: the STR was placed between two flat plate transducers and the radial force was measured while the two plates compressed the device. For this study, the radial force was measured while the STRs were compressed to 50% of their labeled diameter. Compression tests consisted of five consecutive cycling compressions and were repeated three times for each device. The mean value of the force exerted by the machine to compress the STR at each cycle was used to calculate the radial force (F) per unit length (L) expressed in N/mm as follows: F (average)/L (after compression).
Pull up traction test
The Pull up traction test aimed to evaluate how the outward radial pressure exerted by the STRs varied during retrieval along tubes of different diameters. Tests were performed using a tensile test machine as follows: the device was deployed within a silicone tube maintained by a rigid scaffold and the push wire was connected to the traction transducer arm (figure 1). Silicon tubes were previously filled with saline solution heated to 37°C. The transducer arm retrieved the devices with a velocity of 2 mm/s for a distance of 5 cm. The force F exerted by the arm during the retrieval was measured in newtons. Therefore, the average of these values (obtained after 10 tractions) was used to derivate the radial pressure RN exerted by the stent toward the vessel wall. This test was conducted using tubes of 1.5 and 3.5 mm inner diameter and was repeated 10 times for each STR. The radial pressure exerted by the stent on the silicone pipe was calculated considering the contact pressure. Owing to the friction, this contact pressure was normal to the internal surface of the silicone pipe but also had a tangential component RT. The total force F measured by the tensile test machine was the sum of tangential contact pressures RT over the entire internal surface of the silicone pipe. Assuming a uniform contact pressure this can be shown as
where r is the internal radius of the silicone pipe and L the length of the deployed stent.
Figure 1.
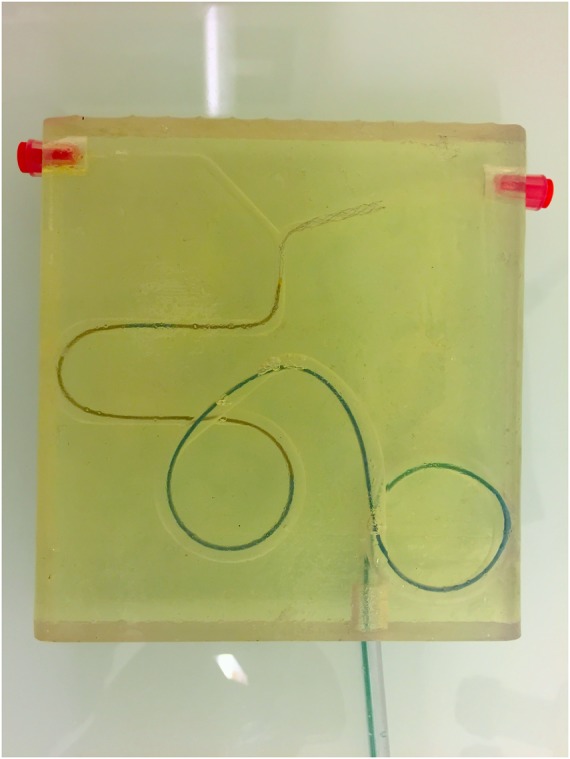
The rigid 3D printed model used for the experiments.
According to the Coulomb friction law, there is a relationship between RN and RT when the stent is sliding: RT=mRN, which depends on the friction coefficient. Finally, the radial pressure RN is obtained as follows
It is expressed in pascals (N/m2). For our calculations, the friction coefficient was not measured; it was chosen as equal to 0.4, according to values given in the paper by Ranc et al.7
Functional tests
Functional tests aimed to visually evaluate the ability of an STR' to remain in close apposition to the wall of the vessel and to maintain the thrombus engaged within its struts during the retrieval. Tests were performed using a rigid 3D printed vascular model reproducing the brain anterior circulation (figure 2). Artificial vessels were produced with realistic dimensions but magnified tortuosity in order to evaluate the apposition of the device in unfavorable conditions; the diameter of the middle cerebral artery of the model measured 2.5 mm, the terminal carotid 3 mm, from the carotid syphon to the cervical carotid segment the diameter increased from 4 to 6 mm. During the experiments the model was continuously flushed with saline solution previously heated at 37°C in order to allow the optimal expansion of devices made of nitinol alloy. Two authors with experience in thrombectomy procedures (PM and VC) performed the functional tests, each experiment was filmed and two other authors (FJ and DA) also visually analyzed the results.
Figure 2.

‘Pull up traction test’ setting: the figure shows a stent retriever deployed within a silicone tube maintained by a rigid scaffold; the push wire is connected to the traction transducer arm of the tensile test machine.
Retrieving test
The Retrieving test was conceived to visually evaluate the ability of an STR' to remain in close apposition to the wall of the vessel during the retrieval. STRs were delivered within the middle cerebral artery of the model. A few minutes after deployment, allowing for their complete expansion, devices were gently retrieved along the vascular model to the guiding catheter connected to the model. The test was repeated 10 times for each stent.
In vitro thrombectomies
This test was aimed to visually evaluate the ability of an STR' to maintain the thrombus engaged within its struts during the retrieval. In order to identify any difference in the behavior of an STR' when interacting with thrombi of different features,7 red and white artificial thrombi were employed. In order to ensure that thrombus removal was uniquely due to the device, neither proximal flow arrest nor manual aspiration was performed during retrievals.8 9 Artificial thrombi were placed within the middle cerebral artery or terminal carotid artery of the model. Stent retrievers were delivered across the thrombus using a standard technique.10 The distal extremity of the devices was delivered approximately 1 cm distally to the thrombus. After 3–5 min of embedding time, The STRs were gently retrieved into the guiding catheter. In vitro thrombectomies were conducted with red and white artificial thrombi of three diameters: small (2 mm), middle (4 mm), and large (6 mm). Devices were tested five times for each thrombus type; the rate of successful clot removal was calculated singularly for each clot type and as a whole for ‘red’ or ‘white’ clots.
Thrombi preparation
Thrombi were generated using human blood obtained from 10 healthy volunteers with no history of use of antiplatelet medication or other medication that might interfere with normal coagulation. Each volunteer provided written informed consent before blood samples were obtained.
White ‘stiff’ thrombi
Blood samples were collected into tubes containing 3.2% sodium citrate solution (1:9 in volume) used for blood anticoagulation. In order to obtain platelet-rich plasma,11 tubes containing whole blood were centrifuged at 350 g for 10 min at 22°C. After centrifugation, the plasma layer was extracted for establishment of thrombus while the erythrocyte layer was discarded. CaCl2 was added to the platelet-rich plasma in a ratio of 100 μL:1000 μL to reverse the effect of the sodium citrate. The recalcified plasma was then incubated at 37°C for 1 h. Finally, white thrombi formed from platelet-rich plasma were extracted.
Red ‘soft’ thrombi
Whole blood samples were collected in standard tubes without sodium citrate solution. After collection, tubes were stored at 37°C for 24 h. Subsequently, red thrombi formed from the whole blood were extracted.12
Thrombi flat plate compression test
Flat plate compression tests aimed to evaluate whether stiffness varied between artificial red and white thrombi. Similarly to flat plate tests performed for STRs, thrombi of identical cylindrical shape were placed between two flat plate transducers and the force needed to compress the clots to 50% of their height was measured. Tests consisted of five non-consecutive compression cycles; the mean value of the force exerted by the machine to compress the clot at each cycle was used to calculate the radial force expressed in mN/mm2. White clots were nearly 13 times stiffer than red clots; the force needed to compress white clots was 9 mN/mm2, whereas the force needed to compress red clots was 0.7 mN/mm2.
Statistics
To investigate whether the variations of the radial pressure exerted by the devices within tubes of diameter 1.5 and 3.5 mm, recorded in Pull up traction tests, were statistically significant, a Mann–Whitney test was employed.
The rates of successful clot removal recorded for in vitro thrombectomies using different devices for red and white clots were compared with the McNemar test. Differences were considered statistically significant for p<0.05.
Results
Mechanical tests
The results of Flat plate compression tests and Pull up traction tests are reported in table 2.
Table 2.
Result of Flat plate tests and Pull up traction tests
| Size |
Flat plate tests Radial force density N/mm |
Pull up traction tests Radial pressure Pa (N/m2) (1.5 mm tube) |
Pull up traction tests Radial pressure Pa (N/m2) (3.5 mm tube) |
|---|---|---|---|
| Trevo PV | |||
| 4–20 | 0.01480 | 920 | 50* (p=0.050) |
| 3–20 | 0.00600 | 300 | 60* (p=0.030) |
| Eric | |||
| 3–20 | 0.01100 | 220 | – |
| 4–24 | 0.01850 | 1540 | 340* (p=0.008) |
| 6–44 | 0.01304 | 770 | 400* (p=0.010) |
| Embotrap | |||
| 5–21 | 0.00642 | 1430 | 770* (p=0.014) |
| Separator 3D | |||
| 4.5–26 | 0.00791 | 1360 | 400* (p=0.006) |
| Revive | |||
| 4.5–22 | 0.01269 | 1360 | 850* (p=0.021) |
| Mindframe | |||
| 3–23 | 0.00451 | 1250 | 330* (p=0.034) |
| Solitaire FR | |||
| 4–20 | 0.00448 | 1110 | 530* (p=0.014) |
| 6–30 | 0.00351 | 1060 | 580* (p=0.020) |
| Preset | |||
| 4–20 | 0.00521 | 1090 | 730* (p=0.018) |
| 6–30 | 0.00368 | 630 | 600* (p=0.25) |
| Preset LT | |||
| 3–20 | 0.00320 | 1060 | 160* (p=0.03) |
| 4–20 | 0.00370 | 460 | 480* (p=0.77) |
| Catch | |||
| 3–15 | 0.00350 | 840 | 220* (p=0.049) |
| 4–20 | 0.00444 | 1810 | 360* (p=0.010) |
| 6–30 | 0.00368 | 1870 | 900* (p=0.030) |
*p Value for the comparison between force exerted in tubes of 1.5 and 3.5 mm inner diameter during Pull up traction tests. Note results obtained with Preset 6–30 and Preset LT 4–20 for which there is not significant shift of the radial pressure when retrieved in tubes of different diameter; p=0.25 and p=0.77, respectively.
During Flat plate compression the devices tended to become oval in shape hence discharging radial force toward the axis orthogonal to the plates. This phenomenon was more pronounced for the incomplete section devices (Solitaire FR (Medtronic, Irvine, California, USA), Catch (Balt, Montmorency, France) and Preset (Phenox, Bochum, Germany) in our study) since the free edges of the stent circumferentially overlapped under compression. Consequently, this test was not a reliable comparator for all devices in this study; nevertheless it allowed a separate comparison for complete and incomplete section devices. Among complete section devices, comparing STRs of similar diameter, Eric 4–24 (Microvention, Aliso Viejo, California, USA) had the highest radial force followed by Trevo PV 4–20 (Stryker, Kalamazoo, Michigan, USA), Revive 4.5–22 (Codman, Raynham, Massachusetts, USA), Separator 3D 4.5–26 (Penumbra Inc, Alameda, California, USA), and Embotrap 5–21 (Nauravi, Galway, Ireland) (these last two device showed similar radial force). Among incomplete section devices (Solitaire FR 4–20, Catch 4–20, and Preset 4–20) the value of the radial force density was comparable and it was inferior to the lowest value recorded for complete section devices.
The Pull up traction tests permitted the evaluation of the radial pressure variation when the STRs are retrieved along tubes of increasing diameter. Among the complete section devices, Eric 4–24/3–20, Trevo PV 4–20/3–20, Mindframe 3–23 (Medtronic, Irvine, California, USA) and Separator 3D 4.5–26 showed significant reduction of the radial pressure when retrieved within tubes of 3.5 mm in comparison with the pressure recorded in tubes of 1.5 mm inner diameter. Similar behavior was recorded for the Catch 3–15/4–20 and Preset LT 3–20, among incomplete section devices (table 2). Only Preset LT 4–20 and Preset 6–30 showed no significant difference in radial pressure shift when retrieved in tubes of 1.5 and 3.5 inner diameter; p=0.77 and p=0.25, respectively.
Functional tests
The Retrieving tests allowed evaluation of the ability of an STR' to remain in close apposition to the wall of the vessel during the retrieval (table 3). For the purpose of this test, devices were subdivided into (1) devices that remained in close apposition to the vessel wall during the entire retrieval; (2) devices that remained in apposition to the vessel wall but showed a degree of elongation around acute vessel angles (figure 3); (3) devices that completely lost contact with the vessel wall during the retrieval. The following devices remained in close apposition to the vessel wall during the entire retrieval: Preset 6–30, Catch 6–30, and Solitaire FR 4–20/6–30. The following devices remained in apposition to the vessel wall but demonstrated elongation when retrieved across acute angles: Embotrap 5–21, Separator 3D 4.5–26, Revive 4.5–22, and Eric 4–24/6–44.
Table 3.
Results of Retrieving tests
| Device | Size | Close apposition | Elongation | Loss of contact |
|---|---|---|---|---|
| Trevo PV | 4–20 | + | ||
| 3–20 | + | |||
| Eric | 3–20 | + | ||
| 4–24 | + | |||
| 6–44 | + | |||
| Embotrap | 5–21 | + | ||
| Separator 3D | 4.5–26 | + | ||
| Revive | 4.5–22 | + | ||
| Mindframe | 3–23 | + | ||
| Solitaire FR | 4–20 | + | ||
| 6–30 | + | |||
| Preset | 4–20 | + | ||
| 6–30 | + | |||
| Preset LT | 3–20 | + | ||
| 4–20 | + | |||
| Catch | 3–15 | + | ||
| 4–20 | + | |||
| 6–30 | + |
Figure 3.
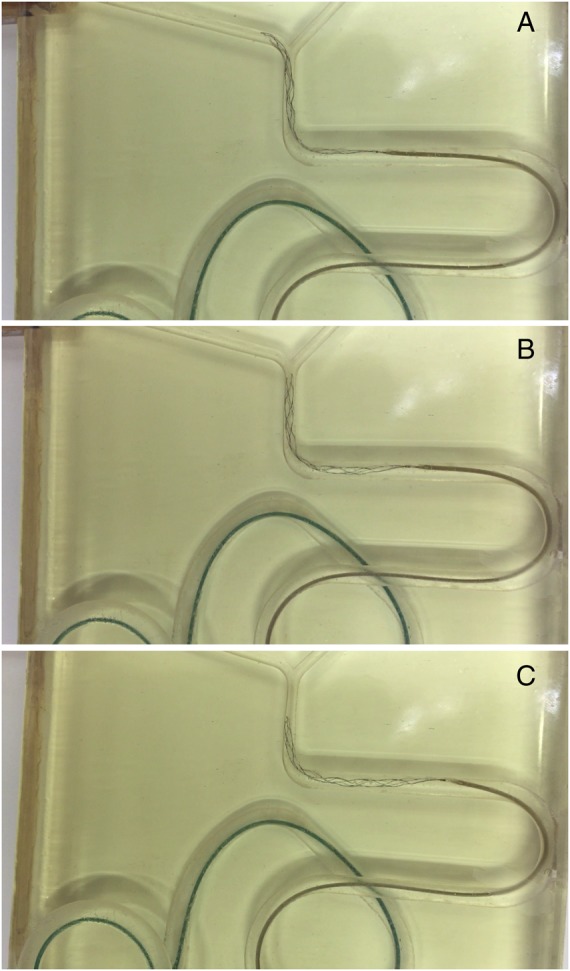
Some devices showed a greater degree of elongation around acute vessel angles: interaction of the proximal, middle, and distal thirds of Solitaire 4–20 (A–C).
The following devices completely lost apposition to the vessel wall during the retrieval: Preset LT 3–20, Eric 3–20, and Mindframe 3–23.
In vitro thrombectomies allowed an appreciation of the interaction between devices and thrombi of different consistency (red or white) and sizes (small, moderate, or large), (table 4).
Table 4.
Results of in vitro thrombectomies
| Device | Size | Large white clot | Medium white clot | Small white clot | % | Large red clot | Medium red clot | Small red clot | % |
|---|---|---|---|---|---|---|---|---|---|
| Trevo PV | 4–20 | 0/5 | 2/5 | 3/5 | 33 | 3/5 | 4/5 | 5/5 | 80 |
| 3–20 | 0/5 | 1/5 | 2/5 | 20 | 3/5 | 3/5 | 3/5 | 60 | |
| Eric | 3–20 | 0/5 | 1/5 | 2/5 | 20 | 3/5 | 3/5 | 2/5 | 53 |
| 4–24 | 0/5 | 2/5 | 3/5 | 33 | 2/5 | 3/5 | 4/5 | 60 | |
| 6–44 | 0/5 | 3/5 | 3/5 | 40 | 2/5 | 5/5 | 4/5 | 73 | |
| Embotrap | 5–21 | 1/5* | 2/5 | 3/5 | 40 | 3/5 | 5/5 | 4/5 | 80 |
| Separator 3D | 4.5–26 | 0/5 | 2/5 | 2/5 | 26 | 2/5 | 2/5 | 3/5 | 46 |
| Revive | 4.5–22 | 1/5* | 2/5 | 3/5 | 40 | 3/5 | 5/5 | 4/5 | 80 |
| Mindframe | 3–23 | 0/5 | 2/5 | 2/5 | 26 | 3/5 | 4/5 | 3/5 | 66 |
| Solitaire FR | 4–20 | 0/5 | 3/5 | 3/5 | 40 | 4/5 | 5/5 | 5/5 | 93‡ |
| 6–30 | 1/5* | 3/5 | 4/5 | 53 | 4/5 | 5/5 | 5/5 | 93‡ | |
| Preset | 4–20 | 0/5 | 3/5 | 4/5 | 46 | 4/5 | 5/5 | 5/5 | 93‡ |
| 6–30 | 2/5* | 3/5 | 4/5 | 60† | 5/5 | 5/5 | 5/5 | 100§ | |
| Preset LT | 3–20 | 0/5 | 1/5 | 2/5 | 20 | 3/5 | 2/5 | 3/5 | 53 |
| 4–20 | 0/5 | 2/5 | 2/5 | 26 | 3/5 | 3/5 | 3/5 | 60 | |
| Catch | 3–15 | 0/5 | 1/5 | 2/5 | 20 | 2/5 | 3/5 | 3/5 | 53 |
| 4–20 | 1/5* | 1/5 | 2/5 | 26 | 3/5 | 3/5 | 3/5 | 60 | |
| 6–30 | 1/5* | 3/5 | 4/5 | 53 | 5/5 | 5/5 | 4/5 | 93‡ |
*Minimal clot displacement.
†The rate of white clot removal found for Preset 6–30 was significantly higher than the rates for Catch 3–15, Preset LT 3–20, Eric 3–20, and Trevo 3–20 (60% vs 20%; p=0.031).
‡The rate of red clot removal found for Solitaire 4–20/6–30, Preset 4–20, and Catch 6–30 was significantly higher than the rates for Separator 3D, Eric 3–20, Preset LT 3–20, and Catch 3–15 (93% vs 46%; p=0.016 or 93% vs 53%; p=0.031).
§The rate of red clot removal found for Preset 6–30 was significantly higher than the rates for Separator 3D (100% vs 46%; p=0.008), Eric 3–20, Preset LT 3–20, Catch 3–15 (100% vs 53%; p=0.016), Trevo 3–20, and Eric 4–24 (100% vs 60%; p=0.031).
With large white thrombi (6 mm), the delivery catheter navigated across the clot by passing between the clot and the vessel wall. Once delivered, stent retrievers remained constrained between the clot and the vessel wall (figure 4). Thereafter, the devices did not expand but slid over the thrombus; only in a few cases was the device effective in a minimal clot displacement. Interacting with a small or moderate size white thrombus (2–4 mm), stents partially expanded after deployment. Nevertheless, as noted in the larger white thrombi, the clot was not penetrated by the stent struts but remained on the side of the devices compressed against the vessel wall. Contrary to larger thrombi, small and middle thrombi were partially engaged by the stent struts and displaced during the retrieval. Nevertheless, the clots did not remain engaged during the entire retrieval but intermittently rolled between the stent and the vessel wall toward the tip of the stent. The effectiveness of the removal was secondary to the clot being maintained by the stent struts and the clot rolled between the stent and the vessel wall. The results of in vitro thrombectomies performed using white thrombi showed a significant difference between the performance of Preset 6–30 and those of Catch 3–15, Preset LT 3–20, Eric 3–20, and Trevo PV 3–20 (60% vs 20%; p=0.031).
Figure 4.
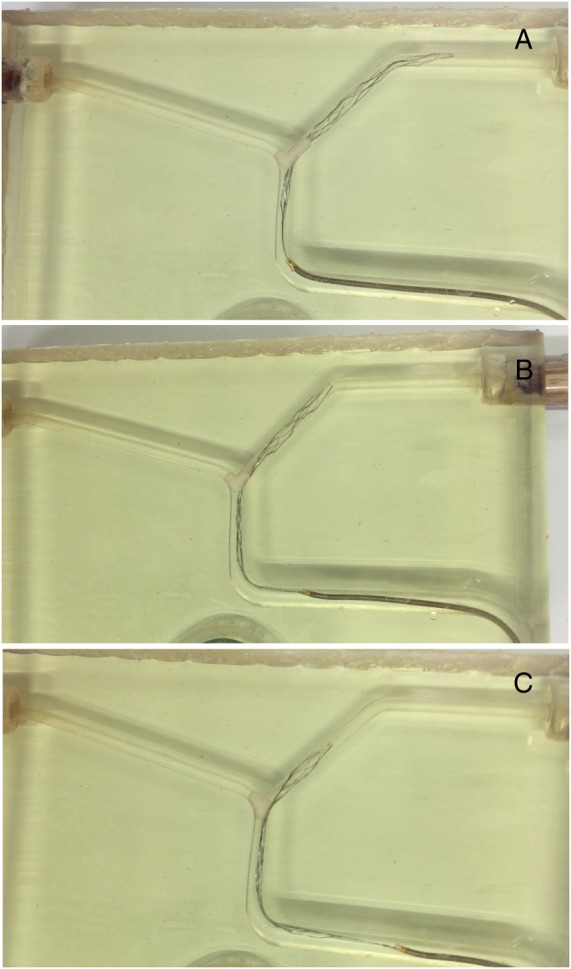
Interaction between Solitaire 4–20 and a large white clot: interaction of the proximal, middle, and distal thirds of the device (A–C).
A completely different interaction with STRs was noted when in vitro thrombectomies were performed with red thrombi. In contrast to white thrombi, the delivery microcatheter navigated through the clot and not alongside it. Furthermore, once deployed, stent retrievers easily penetrated the thrombus with their struts and very often the clot entered within their lumen. Red clots mostly remained engaged with the devices during retrieval, with a higher rate of complete clot removal than recorded for white thrombi. Nevertheless, we noted some differences in embolic complications, which occurred because red thrombi had a tendency to fragmentize during retrievals. Preset 6–30 was effective in red clot removal in 100% of the tests. This rate was significantly higher than the rates obtained with Separator 3D (100% vs 46%; p=0.008), Eric 3–20, Preset LT 3–20, Catch 3–15 (100% vs 53%; p=0.016), Trevo PV 3–20 and Eric 4–24 (100% vs 60%; p=0.031).
Solitaire FR 4–20 and 6–30, Preset 4–20, and Catch 6–30 were effective in red clot removal in 93% of the tests. This rate was significantly higher than the rates of Separator 3D, Eric 3–20, Preset LT 3–20, and Catch 3–15 (93% vs 46%; p=0.016 or 93% vs 53%; p=0.031).
Discussion
Our study allowed the evaluation of mechanical characteristics of different STRs and permitted a better understanding of how they interact with different types of thrombi.
To date, only a few experimental studies investigating the interaction of thrombectomy devices with artificial thrombi have been carried out.13–18 In addition to the device–clot interaction, these studies conducted a comparative evaluation of clot removal efficacy of different thrombectomy devices. In contrast to our study, none of the previous studies evaluated the variation in STR radial pressure during retrieval. Furthermore, these studies predominantly evaluated the interaction of devices with one type of thrombus.
Wenger et al15 evaluated the interaction of four devices (Solitaire, Aperio (Acandis, Pforzheim, Germany), Merci X6, and Merci L5 (Concentric medical, Montain View, California, USA) with artificial clots. The authors found that the migration of the stent struts into the clot is promoted by a strong radial force of the device and by large gaps between filaments. Nevertheless, in our study we found that when delivered within small vessels, across large white clots, devices remain constrained and gaps between filaments did not open. Moreover, devices presenting large gaps between filaments, especially open-cell devices, were more prone to lose contact with the vessel wall when retrieved along sharp angles, disengaging the clot. The authors found that the use of longer STRs improved stable clot engagement. Our results concord with this finding, with the larger devices showing a higher rate of complete clot removal.
Madjidyar et al16 experimentally evaluated device–clot interaction and the influence of distal aspiration on performances of four thrombectomy devices: Solitaire FR, Trevo, Separator 3D, and Aperio. The authors performed experimental thrombectomies, with or without additional distal aspiration, using an artificial vascular model reproducing the human brain anterior circulation. Distal aspiration was performed during retrievals via an intermediate catheter placed at the origin of the middle cerebral artery. The authors found that after stent deployment, the artificial thrombus was pushed against the opposite vessel wall and no integration of the clot into the inner stent lumen could be observed in any device. Moreover, when the stent was pulled back, the clot rolled between the device and the vessel wall and wandered toward the tip of the device. These findings correspond to our observations when analyzing the interaction between devices and small and moderate-sized white clots. Interestingly, the authors noted that when distal aspiration was added to the retrieval process, clot removal efficacy was comparable for all devices. Such results correlate with our observations in this study.
In pull up traction tests conducted in 1.5 mm inner diameter tubes we demonstrated comparable radial pressure (>900 Pa) for the majority of the 4 mm diameter devices. Hence, it can be reasoned that all devices have equal clot engagement in the early phase of the retrieval. This might explain why no differences in clot removal efficacy were found when distal aspiration was associated with experimental thrombectomies. On the contrary, we observed that when thrombectomies are performed without any additional aspiration, each device has a different performance, which is related to its own mechanical characteristics. Mechanical tests gave an overview of each STR radial pressure. Pull up traction tests performed in tubes of 1.5 mm inner diameter found similar radial pressure for devices of similar diameter (4–4.5 mm). Results of tests performed in tubes of 3.5 mm inner diameter showed different behavior for each device. In this case, most of the tested devices showed a definite decrease of the radial pressure in comparison with values recorded in tubes of 1.5 mm. On the contrary, some incomplete section devices showed constant radial pressure in both 1.5 mm and 3.5 mm inner diameter tubes (Preset 4–20 LT and 6–30, p=0.77 and p=0.25, respectively, in table 2). Interestingly, the Retrieving tests did not show any loss of apposition to the vessel wall during retrieval for such devices. Moreover, a relatively high rate of contact loss was recorded for those devices for which Pull up traction tests have shown a sharp decrease of the radial pressure when tested in 3.5 mm inner diameter tubes.
Analyzing the results of in vitro thrombectomies, we noted that devices did not expand when interacting with large white clots (6 mm) but remained constrained between the clot and the vessel wall, and thus during the retrieval, devices slide over the clot without capturing it. None of the devices tested in our study could penetrate and remove large white thrombi (6 mm). Nevertheless, devices could expand and penetrate smaller white thrombi (2–4 mm). During the retrieval, the contact with these clots was not homogeneous but there was a succession of phases in which the clot was engaged by the stent struts and phases in which the clot was disengaged and rolled between the device and the vessel wall.
A completely different interaction was appreciated when devices were tested with red thrombi; in this instance all thrombectomy devices could easily penetrate and incorporate the clot inside their lumen. During the retrieval red clots showed a tendency to fragment and this was related to the occurrence of a higher rate of embolization of distal fragments.
Investigating the interaction devices and thrombi, we found that removal efficacy was related to the device's ability to maintain a constant radial pressure and hence allow gradual expansion and constant apposition during retrieval (eg, Preset 6–30). This reduced the occurrence of distal fragment embolization during the interaction with both white and red thrombi. Such effective behavior was not recorded for devices for which the radial force reduced sharply during retrieval.
Retrieving tests showed that overall devices flattened when facing sharp vessel angles (90° in our model) (figure 3). Devices that after interaction with sharp angles presented a prompt and complete reopening of the proximal and middle thirds showed better cohesion to the vessel wall and stability during retrieval. Such favorable behavior was mainly recorded for incomplete section devices and, in particular, for devices of larger diameters: Preset 6–30, Solitaire FR 6–30, and Catch 6–30. On the other hand, devices that did not promptly re-expand after interaction with sharp angles lost contact with the vessel wall and jumped abruptly toward the downstream vessels. In our experiments this behavior, mainly recorded for complete section devices, was related to whole clot disengagement and fragment embolization.
Our study has several limitations. First, we used a rigid plastic model and hence during retrievals across the curves and tortuosity of a model, the vasculature did not straighten. Nevertheless, we planned to employ a rigid model in order to exacerbate device performance and to assure identical conditions for all devices. Moreover, the thrombus interaction with the inner surface of the plastic vessel is not comparable with the interaction with the human endothelium, consequently the resistance recorded in our experiments during retrieval was lower than in real vessels. In addition, despite of our best efforts to produce clots with similar features, thrombi slightly varied in size or texture.
Conclusions
Our results add some innovative information to the current understanding of mechanical thrombectomy.
All STRs slide over large white thrombi, with the clot failing to be retrieved. To our knowledge, this thrombus removal failure mechanism has not been reported so far.
White, small and medium thrombi are not permanently engaged by the stent struts but roll between it and the vessel wall during retrieval. On the other hand, red thrombi are easily caught by devices but have a tendency to fragment.
Constant radial force during retrieval allows constant apposition to the vessel wall and pressure over the clot. Such features allow for a higher rate of clot removal efficacy.
Larger devices (6 mm in diameter) have better clot removal performance and increase the probability of capturing the clot.
The results of our study have to be compared with further experimental and clinical investigations.
Acknowledgments
The authors gratefully acknowledge Professor Thierry Metens for assistance with statistical analysis.
Footnotes
Contributors: All authors contributed to planning the research and conducting the experiments. PM and FJ edited the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: AB unrelated: consultancy: Covidien (consultant for ev3), Stryker; grants/grants pending: Covidien. VC: consultancy: Balt, Codman Neuro-DePuy Synthes, Stryker, MicroVention; payment for lectures (including service on speakers bureaus): Stryker, Balt; payment for development of educational presentations: Covidien.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We are agreeable to sharing unpublished data with readers if requested.
References
- 1.Berkhemer OA, Fransen PSS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleining TJ, et al. . Endovascular therapy for ischemic stroke with perfusion imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafé A, et al. . Stent retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. . Thrombectomy within 8 hours after symptoms onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 6.KrischeK O, Miloslavski E, Fischer S, et al. . A comparison of functional and physical properties of self-expanding intracranial stents (Neuroform 3, Wingspan, Solitaire, Leo+, Enterprise). Minim Invas Neurosurg 2011;54:21–8. [DOI] [PubMed] [Google Scholar]
- 7.Ranc H, Servais C, Chauvy PF, et al. . Effect of surface structure on frictional behaviour of a tongue/palate tribological system. Tribology Int 2006;39:1518–26. [Google Scholar]
- 8.Boeckh-Behrenc T, Schubert M, Forschler A, et al. . The impact of histological clot composition in embolic stroke. Clin Neuroradiol 2014. doi:10.1007/s00062-014-0347-x [DOI] [PubMed] [Google Scholar]
- 9.Chueh JY, Khun AL, Puri AS, et al. . Reduction in distal emboli with proximal flow control during mechanical thrombectomy. Stroke 2013;44:1396–401. 10.1161/STROKEAHA.111.670463 [DOI] [PubMed] [Google Scholar]
- 10.Machi P, Costalat V, Lobotesis K, et al. . Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg 2012;4:62–6. 10.1136/jnis.2010.004051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turk AS, Frei D, Fiorella D, et al. . ADAPT FAST Study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2104;6:260–4. [DOI] [PubMed] [Google Scholar]
- 12.Katori N, Tanaka KA, Szlam F, et al. . The effect of platelet count on clot retraction and tissue-plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg 2005;100:1781–5. 10.1213/01.ANE.0000149902.73689.64 [DOI] [PubMed] [Google Scholar]
- 13.Ogata J, Yutani C, Otsubo R, et al. . Heart and vessel pathology underlying brain infarction in 142 stroke patients. Ann Neurol 2008;63:770–81. 10.1002/ana.21401 [DOI] [PubMed] [Google Scholar]
- 14.Wenger KJ, Berkefeld J, Wagner M, et al. . Flat panel detector computed tomography for the interaction between contrast-enhanced thrombi and stent retrievers in stroke therapy: a pilot study. Clin Neuroradiol 2014;24:251–4. [DOI] [PubMed] [Google Scholar]
- 15.Wenger KJ, Nagl F, Wagner M, et al. . Improvement of stent retriever design and efficacy of mechanical thrombectomy in a flow model. Cardiovasc Intervent Radiol 2013;36:192–7. 10.1007/s00270-012-0420-2 [DOI] [PubMed] [Google Scholar]
- 16.Madjidyar J, Hermes J, Freitag-Wolf S, et al. . Stent-thrombus interaction and the influence of the aspiration on mechanical thrombectomy: evaluation of different stent retrievers in a circulation model. Neuroradiology 2015;57:791–7. 10.1007/s00234-015-1526-4 [DOI] [PubMed] [Google Scholar]
- 17.Mordasini P, Brekenfeld C, Byrne J, et al. . Experimental evaluation of immediate recanalization effect and recanalization efficacy of a new thrombus retriever for acute stroke treatment in vivo. AJNR A J Neuroradiol 2013;34:153–8. 10.3174/ajnr.A3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralla J, Schroth G, Remonda L, et al. . Mechanical thrombectomy for acute ischemic stroke thrombus-device interaction, efficiency, and complications in vivo. Stroke 2006;37:3019–24. [DOI] [PubMed] [Google Scholar]


