Abstract
Objective(s):
Present study was performed in order to uncover new aspects for nicotine-induced damages on spermatogenesis cell lineage.
Materials and Methods:
For this purpose, 36 mature male Wistar rats were divided into three groups as; control-sham (0.2 ml, saline normal, IP), low dose (0.2 mg/kg BW-1, IP) nicotine-received and high dose (0.4 mg/kg BW-1, IP) nicotine-received groups. Following 7 weeks, the expression of bcl-2, p53 and caspase-3 at mRNA and protein levels were investigated by using reverse-transcriptase PCR (RT-PCR) and immunohistochemical (IHC) analyses, respectively. Moreover, the serum level of FSH, LH and testosterone were evaluated. Finally, the mRNA damage was analyzed by using special fluorescent staining.
Results:
Nicotine, at both dose levels, decreased tubular differentiation, spermiogenesis and repopulation indices and enhanced cellular depletion. Animals in nicotine-received groups exhibited a significant (P<0.05) reduction at mRNA and protein levels of bcl-2. More analyses revealed a remarkable (P<0.05) enhancement in expression of p53 and caspase-3 in comparison to control-sham animals. Finally, nicotine resulted in a significant (P<0.05) reduction in serum level of testosterone and elevated mRNA damage.
Conclusion:
Our data showed that, nicotine by suppressing the testosterone biosynthesis, reducing mRNA and protein levels of bcl-2 and up regulating the p53 and caspase-3 mRNA and protein levels adversely affects the spermatogenesis and results in cellular depletion.
Keywords: Apoptosis, Bcl-2, Caspase-3, Nicotine, p53, Testis, Testosterone
Introduction
Various chemicals and drugs are found to adversely affect male reproductive system. For instance, smoking is implicated as potential cause of infertility in males. In the line with this issue, the nicotine has been known to representing 90% of total alkaloid in cigarette. Wide range of population is exposed to nicotine via different routes, including smokeless tobacco products, chewing tobacco and/or smoking. Moreover, due to antiherbivore effect of nicotine, it is widely used as an insecticide and nicotine analogue such as imidaclopride (1). However, very early studies have shown that, the nicotine is toxic substance, which is highly/rapidly absorbed through respiratory system, oral mucosa as well as skin (2, 3). Accordingly, it has been reported that, nicotine inhibits follicular stimulating hormone (FSH) and luteinizing hormone (LH) release from pituitary gland (4). Additional research by Yeh et al (5), showed that, the nicotine as well as cotinine (metabolite of nicotine) decrease rostenedione and testosterone concentrations in rats by competive inhibition of multiple stages in testosterone biosynthesis. More recent studies have shown that, the nicotine obviously affects spermatogenesis (6, 7), significantly reduces the semen quality and adversely affects hypophysis-gonadal hormone axis (8, 9). Moreover, Nesseim et al (10) illustrated that nicotine, in a dose and time dependent manner, adversely affects spermatogenesis, which is almost reversible after nicotine withdrawal, even after small doses. In the other study it has been shown that, tobacco extract, depending on dose, pathologically affects the testicular spermatogenesis (11). Taking together, it has been illustrated that, the nicotine adversely impacts male reproductive system. Meanwhile, the main mechanism(s) involving in nicotine-induced damages remained obscure.
Apoptosis or programmed cell death is an active process that is known as an important monitoring system in different tissues. Indeed, apoptosis occurs physiologically at various phases of germ cell development and appears to be a regular feature in 20% of the germinal lineage (12-14). However different evidences have shown that, an increased apoptosis ratio results in severe reduction in sperm count and adversely affects semen quality (13, 15). Early key discoveries showed that, two distinguished pathways of intrinsic and extrinsic are involved in apoptosis. The intrinsic pathway, also called mitochondrial pathway, triggers the cascade of caspase (caspase-9, caspase-3) interactions which finally results in apoptosis (16, 17). bcl-2 (B-cell lymphoma-2) gene was discovered at the chromosome translocation breakpoint in B-cell follicular lymphomas (14, 18). The bcl-2 family is consisted of several members, including pro-apoptotic (e. g. Bax, Bak) and anti-apoptotic (e. g. Bcl-2, Bcl-XL, Mcl-1) genes. The bcl-2 mainly interacts in mitochondrial apoptosis pathway, which is named as Bcl-2-regulated pathway, as well (18, 19).
On the other hand, tumor suppressor p53 is known as a guardian of cell cycle which is able to initiate the apoptosis after severe DNA damage. Indeed, the p53 controls germ cells cycle during checkpoints at the G1/S and G2/M borders (20, 21). Accordingly, the p53 is often up-regulated after DNA damage resulting in initiation of apoptosis and/or stimulating repair pathways at G1/S as well as triggering cell cycle arrest at G2/M stage (22).
Caspases as endoproteases, hydrolyze bonds between peptide in a reaction that mainly depends on catalytic cysteine residues in the caspase active site. Caspases are involved in cascade of evidences such as apoptosis and inflammation (23). Accordingly, the caspase family has been classified by their role in various pathways, as those (caspase-3, -6, -7, -8, and -9 in mammals) participating in apoptosis and caspases (caspase-1, -4, -5, -12 in humans and caspase-1, -11, and -12 in mice) involving in inflammation (23, 24).
In the line with previous preliminary findings about the nicotine-induced derangements at reproduction level, the present study was done in order to uncover new aspects from the possible mechanism(s), by which the nicotine is able to adversely impact spermatogenesis process. For this purpose, the mRNA and protein levels of bcl-2, p53 and caspase-3 were analyzed for probable nicotine-induced apoptosis. Moreover, the cellular mRNA damage as well as histological alterations were investigated to show any necrosis in testicular tissue.
Materials and Methods
Chemicals
The 25 ml vial of nicotine was provided from Sigma chemical Co. (Germany). The rabbit anti-mouse primary antibodies for bcl-2, p53 and caspase-3 (Biocare, USA) were purchased from Life-Teb Gen (Tehran, Iran). The RNA extraction kit (Sinapure RNA) was obtained from Sinaclone Co. (Karaj, Iran). Secondary antibody and DAB chromogen kit (ScyTek Laboratories, Inc) was provided by Life Teb Gen Co (Tehran, Iran). Mounting medium for immunohistochemical analysis (VECTASHIELD) was from Vector Laboratories (California, USA). Other used materials were standard commercial laboratory chemicals.
Animals
To follow-up the present study we used 36 mature male Wistar rats. In order to adaptation, the animals were kept in standard plastic cages of dimensions of 50×30×20 cm for one week. The diet and water were given ad libitum and all stress factors were reduced to a minimum. Then, the animals were assigned into three groups as control-sham and test groups. The animals in test group subdivided into two groups, including low dose nicotine-received (0.2 mg/kg BW-1) and high dose nicotine-received (0.4 mg/kg BW-1). The nicotine was diluted in saline normal for obtaining required doses. Indeed, in order to mechanistically analyze the nicotine-induced detrimental impact, the nicotine was administrated intra-peritoneally (10). The injectable saline normal was administrated (0.2 ml, IP) in control-sham group. All necessary ethics were considered during present study and were approved by the ethical committee of Urmia University.
Histological analyses
Following 7 weeks, the testicles were dissected out and fixed in Bouin’s solution for 1 week. Ultimately, testicular tissues were dissected-free from surrounding tissues under high magnification by using stereo zoom microscope (Olympus, Japan). Samples were processed through paraffin embedding and blocks were cut by rotary microtome (MICROM GmbH, Germany) and stained with hematoxylin-eosin (H&E). Then the percentage of tubules with positive tubular differentiation index (TDI), tubules with more than 3-4 cellular layers, the percentage of tubules with positive repopulation index (RI), the percentage of tubules with positive spermiogenesis index (SPI), tubules with intact spermiogenesis as well as tubular diameter, height of germinal epithelium and Leydig cells distribution per one mm2 of the interstitial connective tissue were analyzed.
Immunohistochemical (IHC) staining
Tissue section slides were heated at 60 °C for approximately 25 min in a hot air oven (Venticell, MMM, Einrichtungen, Germany). The tissue sections were de-paraffinized in xylene (2 changes, 5 min for each xylene) and rehydrated using an alcohol gradient (90%, 80%, 70% and 50%). The antigen retrieval process was performed in 10 mM sodium citrate buffer. Immunohistochemical staining was conducted according to the manufacturer’s protocol (Biocare and ScyTek, USA). Briefly, endogenous peroxidase was blocked in a peroxidase blocking solution (0.03% hydrogen peroxide containing sodium acid) for 5 min. Tissue sections were washed gently with phosphate buffer saline (PBS, pH 7.2) and subsequently incubated with bcl-2 (1:500), p53 (1:600) and csapase-3 (1:500) primary antibodies at -4 °C, overnight. The sections were rinsed gently with washing buffer (PBS, PH 7.2) and placed in a buffer bath. The slides were then placed in a humidified chamber with a sufficient amount of streptavidin–HRP (streptavidin conjugated to horseradish peroxidase) in PBS containing an anti-microbial agent. The slides were incubated for 15 min. Subsequently, the tissue sections were rinsed gently in washing buffer and placed in a buffer bath. A DAB chromogen was added to the tissue sections and incubated for 5 min. Then, the sections were counter stained with hematoxylin for 20 sec. After that, the sections were dipped in weak ammonia (0.037 ml) for 10 times, rinsed with distilled water and cover slipped. The bcl-2, p53 and caspase-3-positive cells were count in one mm2 of the tissue and quantitatively compared between groups. Moreover, the cellular distribution was analyzed by software analyses (see next).
Fluorescent analyses for RNA damage
The RNA damage was assessed based on Darzynkiewicz method (25). In brief, the testicular samples were washed out with ethyl-alcohol and cut by cryostat (8 µm). The prepared sections then were fixed by different degrees of ethanol (90%, 80%, 70% and 60%) for 15 min. After that, the sections were rinsed in acetic acid (1%) and then washed with distilled water. The specimens were stained with acridine-orange for 3 min and distained in phosphate buffer (PH 6.85). After that, the slides were followed for fluorescent colors differentiation in calcium chloride. The necrotic follicular cells were characterized by loss of RNA and/or with faint red stained RNA. The normal cells were marked with bright red RNA at the apex of the nuclei. Finally, the stained cells were estimated by using software analyses (see next).
RNA isolation
Total RNA was extracted from testicles of test and control animals based on manufactures order for kit (Sinapure RNA, Iran). Each testis was homogenized by using homogenizer Precellys 24 (Bertin Technologies, Aix-en-Provance, France). Subsequently, the samples were processed according to the manufacturer’s instructions. Isolated RNA was stored at −70 °C. The RNA quality and purity were measured with a NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
For the synthesis of cDNA, 5 µg of purified RNA was used. We added 1 µl DNase I (Invitrogen, Eugene, USA), 1 µl DNase I reaction buffer (Fermentas, Burlington, Canada) and H2O to reach a volume of 10 µl. This mixture was incubated for 30 min at 37 °C in an ATP Thermal Cycler (Arash Pishro Teb, Tehran, Iran). After incubation, 1 µl EDTA (Fermentas) was added and incubation continued at 65 °C for 10 min. Then 30 µl of the reaction mixture (8 µl of reaction buffer for M-MuLV reverse transcriptase (Fermentas, Burlington, Canada), 5 µl 10 mM 4dNTP (Fermentas, Burlington, Canada), 0.3 µl Ribo Lock inhibitor (Fermentas, Burling-ton, Canada), 1 µl oligo (dT) + random primers (Promega, Madison, USA) and 15.7 µl H2O) was added to the samples. The mixture was incubated for 60 min at 42 °C, for 10 min at 70 °C, and at the end was maintained at 4 °C. The obtained cDNA was stored at −20 °C. The PCR conditions were run as follows: general denaturation at 95 °C for 3 min, 1 cycle, followed by 35 cycles of 94 °C for 20 sec; annealing temperature (52 °C for 1 min for p53, 62 °C for 1 min for bcl-2, 50 °C for 30 sec for caspase-3 and 63 °C for 30 sec for GAPDH); elongation: 72 °C for 1 min and 72 °C for 5 min. Specific primers (26-28) were designed and manufactured by CinnaGen (CinnaGen Co Tehran, Iran). Primers pair’s sequences and products size for each individual gene are depicted in Table 1. Final PCR products formed were analyzed on 1.2% agarose gel electrophoresis and densitometry analysis of the bands were done by using PCR Gel analyzing software (ATP, Tehran, Iran). The control was set at 100% and experimental samples were compared to the control.
Table 1.
Sequences of the primer pairs and product sizes used for RT-PCR
| Gene name | primer | product size |
|---|---|---|
| P53 | 5’-ATGGAGGAGTCACAGTCGGATA-3 | 250 bp |
| 5’-GACTTCTTGTAGATGGCCATGG-3’ | ||
| Bcl-2 | 5’ - CGCCCGCTGTGCACCGAGA-3’ | 228 bp |
| 5’ -CACAATCCTCCCCCAGTTCACC-3’ | ||
| Caspase-3 | 5’-TACCCTGAAATGGGCTTGTGT-3’ | |
| 5’-GTTAACACGAGTGAGGATGTG-3’ | 446 bp | |
| GAPDH | 5’-GTTACCAGGGCTGCCTTCTC-3’ | |
| 5’-GGGTTTCCCGTTGATGACC -3’ | 390 bp |
Blood sampling and serum preparation
Blood samples from corresponding animals were collected directly from heart and the serum was separated by centrifugation (3000 g for 5 min) and subjected to assessment for serum luteinizing hormone (LH), follicular stimulating hormone (FSH) and testosterone concentrations. The serum levels of hormones were analyzed by using electrochemi-lunescence method. The intra-assay coefficient variance for LH, FSH and testosterone were, 5.9% (for 10 times), 4.18% (for 10 times) and 4.8% (for 10 times), respectively. Inter-assay coefficients variances of 8 5.9% (for 10 times), 6.9% (for 10 times) and 9.9% (for 10 times) were also calculated for LH and FSH, respectively.
Statistical and Image analyses
The data are represented as the mean±SD for quantitative parametric data; Student’s t-test was used to compare means between groups, and a one-way ANOVA to compare more than two groups. The intragroup difference was assessed using a post hoc test and calculation of the least significant difference. Significance was set at P<0.05. The distribution of bcl-2, p53 and caspase-3-positive cells per one mm2 of the tissue were assessed by using image pro-insight microscope analyses software (version 9:00). Onboard camera (Sony, Japan) was used for image capture photomicrographs. The fluorescent red and green spots for mRNA damage were analyzed by using image pro-insight software (Version 9:00) based on pixel intensity.
Results
General findings
In order to estimate the effect of nicotine on gonadal weight, the total testicular weight relative to total body weight was analyzed. Observations revealed that nicotine, in a dose dependent manner, diminished testicular weight relative to total body weight. More analyses showed a remarkable (P<0.05) reduction in serum level of testosterone in the nicotine-received animals versus those in control group (Table 2).
Table 2.
Effect of nicotine on original body weight, final body weight after study, Absolute weight of testes and relative weight of testes. All data are presented in mean±SD
| Control | 0.2 mg/kg | 0.4 mg/kg | |
|---|---|---|---|
| Original body weight (g) final body weight (g) | 202.2±12.6*
240±15.2* |
216.4±27.9*
200±22.2* |
214.6±9.5*
226±10.3* |
| Absolute testicular weight (g) | 2.210±0.14* | 2.32±0.025* | 1.89±0.032** |
| Relative weight of testes (%) | 0.98±0.52* | 1.01±0.010* | 0.72±0.30** |
Note: versus
(P<0.05)
Nicotine reduced serum levels of LH, FSH and testosterone
Biochemical analyses revealed that, the nicotine diminished serum levels of LH and FSH, dose dependently. Accordingly, the animals in high dose (0.4 mg/kg) nicotine-received group showed a significant (P<0.05) reduction in serum levels of LH and FSH versus control and low dose (0.2 mg/kg) nicotine-received groups. Moreover, the animals in nicotine-received groups exhibited a remarkable (P<0.05) reduction in serum levels of testosterone, LH and FSH in comparison to those in control group (Table 3).
Table 3.
Nicotine-induced alterations at serum levels of follicular stimulating hormone (FSH), luteinizing hormone (LH) and testosterone in different groups. All data are presented in mean±SD
| Control | 0.2 mg/kg | 0.4 mg/kg | |
|---|---|---|---|
| FSH (mIU/ml) | 0.25±0.01* | 0.21±0.01* | 0.19±0.006* |
| LH (mIU/ml) | 0.27±0.005* | 0.23±0.005** | 0.15±0.002*** |
| Testosterone (ng/ml) | 33.39 ±0.53* | 20.16±1.06** | 13.65±4.80*** |
Note:* versus **, (P<0.05), * versus *** (P<0.001) and ** versus ***, (P<0.001)
Nicotine adversely affected spermatogenesis
As preliminary findings, light microscopic analyses showed a significant (P<0.05) reduction in percentage of tubules with positive TDI, RI and SPI in the nicotine-received groups. These impairments were enhanced depending on administrated doses. Moreover, the animals in the nicotine-received groups exhibited a severe edema in interstitial connective tissue, decreased Leydig cells distribution, and diminished tubular diameter as well as reduced spermatid volume. More analyses showed that nicotine, in a dose dependent manner, resulted in tubular depletion and germinal epithelium dissociation (Figure 1). The data for histomorphometric analyses are represented in Table 4.
Figure 1.
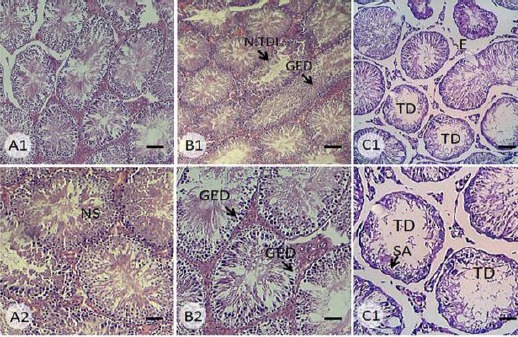
Cross section from seminiferous tubules of (A) control-sham, (B) 0.2 mg/kg nicotine-received and (C) 0.4 mg/kg nicotine-received groups. See intact seminiferous tubules in control group with normal spermatogenesis (NS) and no edema in interstitial connective tissue. Note seminiferous tubule with negative TDI (N.TDI), germinal epithelium dissociation (GED) in cross section from 0.2 mg/kg nicotine-received group, which is developed to complete tubular depletion (TD) and spermatogenesis arrest (SA) in 0.4 mg/kg nicotine-received animals. H&E staining, Scale bars for first row: 120 µm and scale bars for sec row: 50 µm
Table 4.
Mean percentage of seminiferous tubules with positive tubular differentiation, spermiogenesis and repopulation indices in different groups. All data are presented in mean±SD
| control | 0.2 mg/kg | 0.4 mg/kg | |
|---|---|---|---|
| Tubular differentiation Index (%) | 90.24±3.56* | 82.35±4.93** | 63.41±5.71*** |
| Spermiogenesis Index (%) | 75.31±6.33* | 53.11±6.80** | 48.37±4.33** |
| Repopulation Index (%) | 82.45±4.31* | 63.34±2.77** | 40.20±3.61*** |
Note:* versus **, (P<0.05), * versus *** (P<0.001) and ** versus ***, (P<0.001)
Nicotine increased the p53 and caspase-3 expression
Observations revealed a significant (P<0.05) enhancement in p53 expression at mRNA and protein levels. Accordingly, the nicotine resulted in increased distribution of p53-positive cells in one mm2 of the testicular tissue and enhanced the mRNA level of p53, especially at high dose (0.4 mg/kg) level (Figure 2 and 3). More analyses for caspase-3 revealed a remarkable (P<0.05) upregulation in protein level of caspas-3 in nicotine-received groups versus control-sham animals.
Figure 2.
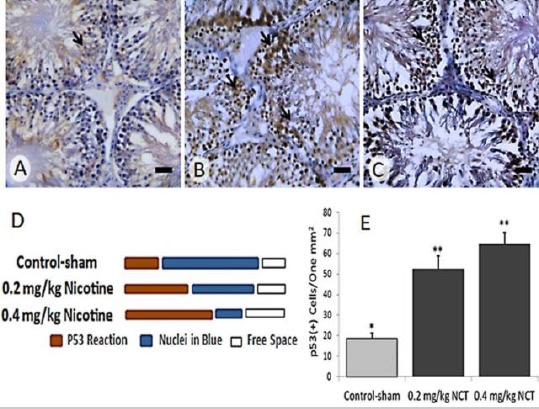
Immunohistochemical staining for p53 in (A) Control-sham, (B) 0.2 mg/kg nicotine-received and (C) 0.4 mg/kg nicotine-received groups. See increased protein level of p53 (brown chromogen sites, arrows) in nicotine-received groups versus control-sham group, Scale bars: 40 µm. (D) semi-quantitative analyze for p53 reaction as positive pixel diagnosis and (E) quantitative p53-positive cells count per one mm2 of the testicular tissue; All data are represented in mean±SD, *versus **, (P<0.001)
Figure 3.
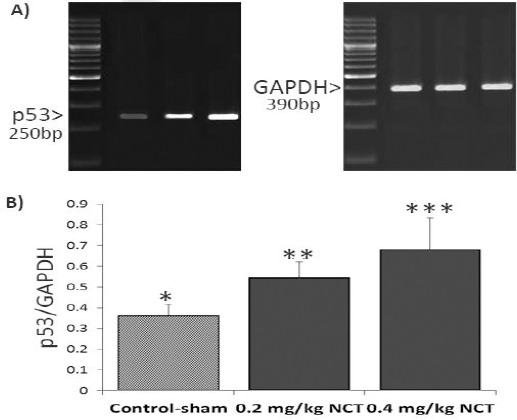
Effect of nicotine (NCT) on mRNA level of p53; (A) the mRNA levels of p53 and GAPDH were evaluated by using semi-quantitative RT-PCR. (B) Represents the density of p53 mRNA levels in testicular tissue that were measured by densitometry and normalized to GAPDH mRNA expression level. Results are expressed as integrate intensity value (IDV) of p53 mRNA level, * versus **, (P<0.001)
Indeed, the protein level of caspase-3 was increased at both mRNA and protein levels, dose dependently (Figure 4 and 5).
Figure 4.
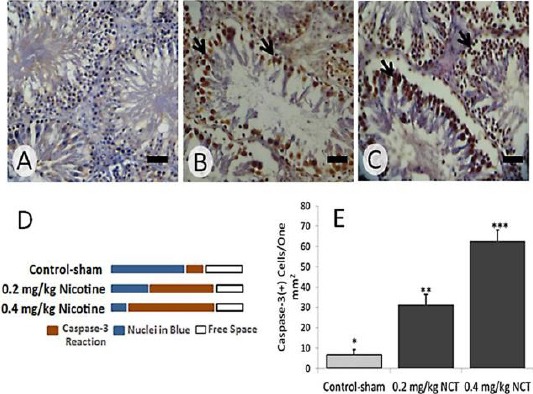
Immunohistochemical staining for caspase-3 in (A) Control-sham, (B) 0.2 mg/kg nicotine-received and (C) 0.4 mg/kg nicotine-received groups. See intensive elevation in protein level of caspase-3 (brown chromogen sites, arrows) in nicotine-received groups compared to control-sham group, Scale bars: 40 µm. (D) semi-quantitative analyze for caspase-3 reaction as positive pixel diagnosis and (E) quantitative caspase-3-positive cells distribution per one mm2 of the testicular tissue; All data are represented in mean±SD, * versus *, (P <0.001), * versus ***, (P<0.001) and ** versus ***, (P <0.05)
Figure 5.
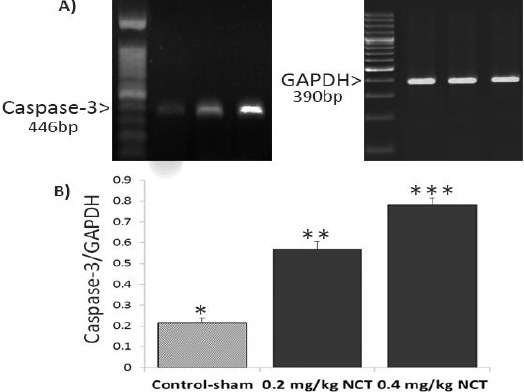
Effect of nicotine (NCT) on mRNA level of caspase-3; (A) the mRNA levels of caspase-3and GAPDH were evaluated by using semi-quantitative RT-PCR. (B) represents the density of caspase-3mRNA levels in testicular tissue that were measured by densitometry and normalized to GAPDH mRNA expression level. Results are expressed as integrate intensity value (IDV) of caspase-3mRNA level, * versus **, (P<0.001), * versus ***, (P<0.001) and ** versus ***, (P<0.05)
The expression of bcl-2 was diminished in nicotine-received groups
Considering the anti-apoptotic role of bcl-2, the protein and mRNA levels of bcl-2 were analyzed. The nicotine, in a dose dependent manner, significantly (P<0.05) reduced the bcl-2 protein level. Accordingly, the animals in high dose nicotine-received (0.4 mg/kg) group exhibited lowest bcl-2-positive cells/one mm2 of the testicular tissue versus the lower dose nicotine-received (0.2 mg/kg) and control-sham groups. The results for RT-PCR corroborated the IHC staining and exhibited a remarkable (P<0.05) reduction at mRNA level (Figure 6).
Figure 6.

Immunohistochemical staining for bcl-2 in (A) control-sham, (B) 0.2 mg/kg nicotine-received and (C) 0.4 mg/kg nicotine-received groups. The protein level of bcl-2 (see brown sites, arrows) is elevated in nicotine-received animals compared to control-sham group, Scale bars: 40 µm. (D) semi-quantitative analyze for bcl-2 reaction as positive pixel diagnosis and (E) Quantitative bcl-2-positive cells distribution per one mm2 of the testicular tissue; All data are represented in mean±SD, * versus **, (P<0.001), * versus ***, (P<0.001) and ** versus ***, (P<0.05)
Nicotine resulted in severe mRNA damage
Special fluorescent staining was done in order to analyze the mRNA damage (albeit in necrotic cells). The nicotine, dose dependently, increased the mRNA damage at spermatogenesis cell lineage. Accordingly, the animals in the high dose nicotine-received (0.4 mg/kg) group exhibited significantly (P<0.05) higher (40.26±3.53) percentage of tubules with mRNA damage versus those animals in lower (0.2 mg/kg) dose nicotine-received (32.19±4.21) and control-sham (13.73±1.26) groups (Figure 7).
Figure 7.

Effect of nicotine (NCT) on mRNA level of bcl-2; (A) the mRNA levels of bcl-2 and GAPDH were evaluated by using semi-quantitative RT-PCR. (B) represents the density of bcl-2 mRNA levels in testicular tissue that were measured by densitometry and normalized to GAPDH mRNA expression level. Results are expressed as integrate intensity value (IDV) of bcl-2 mRNA level, * versus **, (P<0.001), * versus ***, (P<0.001) and ** versus ***, (P<0.05)
Figure 8.

Fluorescent staining for mRNA damage in (A) control-sham, (B) 0.2 mg/kg nicotine-received and (C) 0.4 mg/kg nicotine-received groups. The cross section from control-sham animals is representing intact cells with normal mRNA with red-fluorescent appearance (Arrows). However, note a significant enhancement in mRNA damage at germinal cell levels in nicotine-received animals (yellowish-fluorescent, thick arrows). (D) semi-quantitative analyze for mRNA and (E) percentage of tubules with mRNA damage, all data are presented in Mean±SD, * versus **, (P<0.001)
Discussion
Our data showed that, the nicotine at both dose levels resulted in severe histological damages at spermatogenesis level and diminished serum level of testosterone. Accordingly, nicotine adversely affected the differentiation, repopulation and spermiogenesis indices. Our data showed that, the nicotine upregulated the p53 and caspase-3 expression at both mRNA and protein levels versus control-sham animals. Moreover, observations revealed a significant reduction at mRNA and protein levels of bcl-2 in nicotine-received animals. Finally, we showed that, the nicotine resulted in a remarkable mRNA damage in spermatogenesis cell lineage. Accordingly, the percentage of seminiferous tubules with mRNA damage was increased in the nicotine-received groups.
As preliminary findings, we showed that the nicotine reduced the testicular weight gain relative to total body weight, which could be attributed to testosterone withdrawal. Indeed, it has been shown that, the physiologic interaction between testosterone and Sertoli cells promotes the spermatogenesis process. Therefore, decreased level of the testosterone in the nicotine-received groups could adversely affect the spermatogenesis process (29, 30), which in turn was able to decrease tubular cellularity. Therefore, the cellular depletion resulted in severe reduction in total testicular weight gain. Our findings were in accordance with previously reports for nicotine-exposed animals (6, 7). In the line with this issue, it has been shown that, the nicotine-reduced gonadotropins secretion is partially responsible for sharp reduction in testosterone level as well as Sertoli cells damage (9, 8). Here in present study we showed that, the nicotine decreased the Leydig cells distribution, diminished serum level of LH and FSH as well as testosterone versus control animals. Although defected hypophysis-gonadal hormone axis and diminished testicular endocrine status are illustrated as possible mechanism for nicotine-induced damages, the exact mechanism(s) by which nicotine affects the spermatogenesis process is remained unknown. For this purpose, aside the roles of gonadotropins and testosterone, the role of apoptosis was investigated here in present study as novel aspect for nicotine-induced derangements in testes.
Our RT-PCR and IHC analyses showed that, the nicotine significantly decreased the bcl-2 mRNA and protein levels. Actually, the protein bcl-2 is actively involved in apoptotic pathways. Accordingly, miss expressing of bcl-2 up-regulates the permeability of mitochondrial membrane, which in turn results in intensive release of cytochrome C from mitochondrial inner-membrane space into the cytoplasm (17). Moreover, sequestration of pro-caspases and inhibiting self-cleavage of caspases are known as another possible mechanism for anti-apoptotic activity of bcl-2 (17, 19). Thus, we can hypothesis that, decreased expression of the bcl-2 may trigger the apoptosis pathway partially by up-regulating permeability of mitochondrial membrane and/or by enhancing self-cleavage of caspases (caspase-9).
On the other hand, the interaction between bcl-2 family and the p53 should not be ignored. In order to illustrate the cross link between the bcl-2 and p53 proteins, the mRNA and protein levels of the p53 were analyzed in present study. In fact, the p53 directly impacts the activity of bcl-2 as a part of transcription-independent program of cell death (31). Actually, the cytosolic p53 binds to pro-apoptotic (Bax and Bak) bcl-2 family proteins, which in turn leads to enhancing the permeability of mitochondrial membrane (32, 33). Thus, overexpressed p53, itself, acts as antagonist of anti-apoptotic (bcl-2 and bcl-xL) bcl-2 proteins (27, 28). Our observations revealed a significant enhancement in distribution of p53-positive cells in one mm2 of the tissue. Moreover, the animals in nicotine-received groups exhibited elevated mRNA levels of the p53. Thus, we can come close to this fact that, the nicotine by up regulating the p53 expression promotes the apoptosis pathway in spermatogenesis cell lineages. In the line with this issue, the p53 is a sequence-specific transcription factor, which is activated by diverse forms of cellular stress and is known to mediate the cycle arrest in response to cellular stress (21, 22). In corroboration with this characteristic of p53, the animals in nicotine-received groups showed spermatogenesis arrest (See depleted seminiferous tubules in Figure 1) and increased percentage of seminiferous tubules with negative TDI, RI and SPI indices (Note Table 1). However, it should be considered that the nicotine, even at dose level of 0.4 mg/kg, was able to reduce the TDI, SPI and RI indices approximately 30%-40% of tubules, which is not effective enough to induce complete infertility. To simulate this condition with human cases we can come close to this fact that, although the nicotine adversely impacts the spermatogenesis, spermiogenesis as well as cellular repopulation indices, to see remarkable effects higher doses of nicotine and/or longer time is required.
Caspases are actively involved in cleaving the proteins at aspartic acid residues and are known to be participated in cleaving neighboring amino acids (34, 23). Indeed, once caspases are activated, there seems to be an unalterable commitment to cell death (23, 34). Therefore, here in present study we aimed to investigate the expression of caspase-3, as an executioner caspase, following exposure to the nicotine. Observations revealed a significant enhancement in expression of caspase-3 at both levels of mRNA and protein versus control-sham animals. The caspase-3 activates cytoplasmic endonucleases that degrade the nuclear materials and proteins as well as cytoskeletal proteins (35, 23). Previous studies have shown that, the caspase-3 by cleaving the inhibitor of endonuclease caspase activated DNAse (CAD), results in releasing of CAD. Finally, released CAD degrades chromosomal DNA (36). Considering all these findings, it would be logic to conclude that, nicotine by triggering the apoptotic pathway (by down-regulating bcl-2 expression and by upregulating p53 level) results in caspase-3 overexpression (Note Figure 9). Ultimately, the expressed caspase-3 in turn enhances the protein, DNA and cytosolic materials degradation. Reduced cellularity as well as arrested spermatogenesis in nicotine-received animals confirms this hypothesis.
Figure 9.

Possible pathway for DNA fragmentation in nicotine-received groups; Indeed, reduced expression of bcl-2 results in oligomerization of the Bax proteins in mitochondrial membrane, which leads to cytochrome C (cyt c) linkage in cytoplasmic. Then, by expression/activation of caspase-9 the caspase-dependent pathway initiates, which ultimately triggers caspase-3 overexpression. Elevated caspase-3 expression finally results in severe DNA damage at germinal cell level
It has been shown that non coding RNAs are rapidly and globally degraded during apoptosis. Although the mRNA is stabilized for 7 hr in haploid cells (37), the mRNA decay is triggered early in apoptosis. Indeed the mRNA degradation depends on the permeablization of mitochondrial outer membrane and it is amplified by caspase activation (38, 39). Therefore, we can suggest that nicotine-induced RNA damage correlates with intensive caspase expression. Minding this hypothesis we can conclude that, nicotine exerts its detrimental impact not only by enhancing DNA damage via initiating apoptosis but also it results in sever RNA damage at spermatogenesis lineage levels. On the other hand, it should be considered that nicotine-induced cellular damage results in increased levels of reactive oxygen species (40, 41), which in turn are known to adversely impact the cellular DNA, RNA, protein and lipid structures (42). Considering these findings we can come close to this fact that, aside nicotine-increased caspase-3 expression, the pathologically produced oxidative stress may partially upregulated the RNA damage in nicotine-received testes.
Conclusion
our data showed that, the nicotine by, a- down regulating serum level of testosterone and LH, b- reducing the bcl-2 expression, c-enhancing the p53 and caspase-3 expression and d- by inducing severe RNA damage at germinal epithelium level pathologically affects spermatogenesis. Ultimately, arrested spermatogenesis in turn results in severe reduction in testicular weight gain relative to total body weight.
Acknowledgment
Current paper is a part of thesis NO: 2D-283 which is supported by Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. Authors wish to thank Department of Comparative Histology & Embryology, Faculty of Veterinary Medicine for Laboratory and technical supports.
References
- 1.Ibukun Oyeyipo IP, Raji Y, Benjamin Emikpe O, Bolarinwa AF. Effects of nicotine on sperm characteristics and fertility profile in adult male rats: a possible role of cessation. J Reprod Infertil. 2011;12:201–207. [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. Br Med J. 1975;4:313–316. doi: 10.1136/bmj.4.5992.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell MA, Jarvis MJ, Devitt G, Feyerabend C. Nicotine intake by snuff users. Br Med J. 1981;283:814–817. doi: 10.1136/bmj.283.6295.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake CA. Paradoxical effects of drugs acting on the CNS on the preovulatory release of pituitary LH in porestrous rats. Endocrinology. 1978;79:319–326. doi: 10.1677/joe.0.0790319. [DOI] [PubMed] [Google Scholar]
- 5.Yeh J, Barbieri RL, Friedman AJ. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. J Steroid Biochem. 1989;33:627–630. doi: 10.1016/0022-4731(89)90051-4. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa T, Tawadrous G, Roaia MM, Amer MK, Kader RA, Aziz A. Effect of smoking on seminal plasma ascorbic acid in infertile and fertile males. Andrologia. 2006;38:221–224. doi: 10.1111/j.1439-0272.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadnia H, Ghanbari M, Moradi MR, Khaje-Dalouee M. Effect of cigarette smoke on spermatogenesis in rats. Urol J. 2007;4:159–163. [PubMed] [Google Scholar]
- 8.Hassan A, Abo-Azma SM, Fayed S, Mostafa T. Seminal plasma cotinine and insulin-like growth factor-I in idiopathic oligoasthenoteratozoospermic smokers. Br J Urol Int. 2009;103:108–111. doi: 10.1111/j.1464-410X.2008.07929.x. [DOI] [PubMed] [Google Scholar]
- 9.Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;25:89–97. doi: 10.1016/s0741-8329(01)00176-8. [DOI] [PubMed] [Google Scholar]
- 10.Nesseim WH, Haroun HS, Mostafa E, Youakim MF, Mostafa T. Effect of nicotine on spermatogenesis in adult albino rats. Andrologia. 2010;43:398–404. doi: 10.1111/j.1439-0272.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 11.Gambo IM, Galam NZ, Adamu G, Ayaka LO, Mohammed MB, Ahmed MR, et al. The effect of aqueous leave extract of Nicotiana tabacum (tobacco) on some reproductive parameters and micro anatomy of the testis in male albino wistar rats. J Natl Sci Res. 2013;3:137–143. [Google Scholar]
- 12.Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Peprod. 2002;66:950–958. doi: 10.1095/biolreprod66.4.950. [DOI] [PubMed] [Google Scholar]
- 13.Yin Y, Stahl DE, Wolf BC, Morgentaler A. P53 and Fas are sequential mechanisms of testicular germ cell apoptosis. J Androl. 2002;23:64–70. doi: 10.1002/jand.2002.23.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci. 2012;1:746–754. doi: 10.2741/415. [DOI] [PubMed] [Google Scholar]
- 15.Aitken RJ, Mark AB. Causes and consequences of apoptosis in spermatozoa;contributions to infertility and impacts on development. Int J Dev Biol. 2012;57:265–272. doi: 10.1387/ijdb.130146ja. [DOI] [PubMed] [Google Scholar]
- 16.Hakem R, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 17.Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 18.Borner C. The bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 19.Richard J, Youle AS. The bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 20.Agarwall ML, Agarwall A, Taylore WR, Stark GR. P53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puzio-Kuter AM. The Role of p53 in metabolic regulation. Genes Cancer. 2011;2:385–391. doi: 10.1177/1947601911409738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, He Y, Dubois W, Wu X, Shi J, Huang J. Distinct regulatory mechanisms and functions for p53-activated and p53-repressed DNA damage response genes in embryonic stem cells. Mol Cell. 2012;46:30–42. doi: 10.1016/j.molcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mc Ilwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP (L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darzynkiewicz Z. Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine-orange. Methods Cell Biol. 1990;33:285–298. doi: 10.1016/s0091-679x(08)60532-4. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Falsey RR, Gaitonde S, Sotello V, Kislin K, Martinez JD. Genetic analysis of p53 nuclear importation. Oncogene. 2007;26:7885–7893. doi: 10.1038/sj.onc.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleimani Asl S, Farhadi MH, Moosavizadeh K, Samadi Kuchak Saraei A, Soleimani M, Jamei SB, et al. Evaluation of Bcl-2 family gene expression in hippocampus of 3, 4-methylenedioxymethampheta-mine treated rats. Cell J. 2012;13:275–280. [PMC free article] [PubMed] [Google Scholar]
- 28.Kermera P, Klöckera N, Labesa M, Thomsen S, Srinivasan A, Bähr M, et al. Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett. 1999;453:361–364. doi: 10.1016/s0014-5793(99)00747-4. [DOI] [PubMed] [Google Scholar]
- 29.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 30.Zhang QX, Zhang XY, Zhang ZM, Lu W, Liu L, Li G, et al. Identification of testosterone-/androgen receptor-regulated genes in mouse Sertoli cells. Asian J Androl. 2012;14:294–300. doi: 10.1038/aja.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erster S, Moll UM. Stress-induced p53 runs a transcription-independent death program. Biochem Biophys Res Commun. 2005;331:843–850. doi: 10.1016/j.bbrc.2005.03.187. [DOI] [PubMed] [Google Scholar]
- 32.Petros AM, Gunasekera A, Xu N, Olejniczak ET, Fesik SW. Defining the p53 DNA-binding domain/Bcl-x (L)-binding interface using NMR. FEBS Lett. 2004;559:71–174. doi: 10.1016/S0014-5793(04)00059-6. [DOI] [PubMed] [Google Scholar]
- 33.Talos F, Petrenko O, Mena P, Moll UM. Mitochondrially targeted p53 has tumor suppressor activities in vivo. Cancer Res. 2005;65:9971–9981. doi: 10.1158/0008-5472.CAN-05-1084. [DOI] [PubMed] [Google Scholar]
- 34.Sakai W, Suqasawa K. FANCD2 is a target for caspase 3 during DNA damage-induced apoptosis. FEBS Let t. 2014;588:3778–3785. doi: 10.1016/j.febslet.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Nakada S, Ishiko T, Utsugisawa T, Datta R, Kharbanda S, et al. Role for caspase- mediated cleavage of Rad51 in induction of apoptosis byDNA damage. Mol Cell Cell Biol. 1999;4:2986–2997. doi: 10.1128/mcb.19.4.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tani H, Mizutani R, Salam KA, Tano K, Ijiri K, Wakamatsu A, et al. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 2012;22:947–956. doi: 10.1101/gr.130559.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen B, Goodman HM. Uridine addition after microRNA-directed cleavage. Science. 2004;306:997. doi: 10.1126/science.1103521. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Liu X, Whangbo J, McCrossan G, Sanborn KB, Basar E, et al. Apoptosis Triggers Specific, Rapid, and Global mRNA Decay with 38242Uridylated Intermediates Degraded by DIS3L2. Cell Report. 2015;11:1079–1089. doi: 10.1016/j.celrep.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh RA, Agrawal A, Sharma RK, Nelson DR, Thomas AJ, Jr, et al. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78:491–499. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 41.Jana K, Samanta PK, Kumar D. Nicotine diminishes tresticular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: possible influence on pituitary gonadotropins and alteration of testicular antioxi-dant status. Toxicol Sci. 2010;116:647–659. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 42.Khosravanian H, Razi M, Farokhi F, Khosravanian N. Simultaneous administration of dexamethasone and vitamin E reversed experimental varicocele-induced impact in testicular tissue in rats;correlation with Hsp70-2 chaperone expression. Int Braz J Urol. 2015;41:773–790. doi: 10.1590/S1677-5538.IBJU.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]


