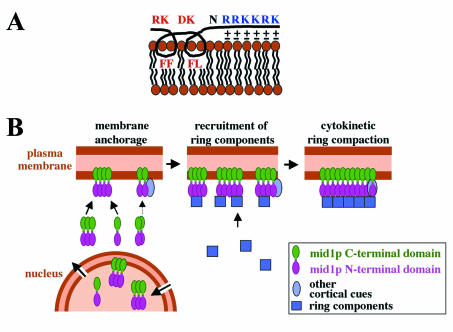
FIG. 9.
Model for mid1p anchoring to the cortex and function. (A) Schematic association of mid1p amphipathic helix and NLS with phospholipids of the plasma membrane. Hydrophobic lateral chains of F and L residues could establish hydrophobic interactions inside one leaflet of the lipid bilayer, whereas basic lateral chains of R and K residues could interact electrostatically with head groups of acidic phospholipids. (B) Functional model for mid1p. Oligomers of mid1p that assemble in the cytoplasm or in the nucleus are recruited to the plasma membrane (or the nuclear envelope) by interactions between the C-terminal domain and phospholipids. Additional recruitment to membranes may be mediated by mid1p N-terminal domain, possibly by interacting with other cortical cues. In early mitosis, mid1p oligomers activated by plo1p phosphorylation form a “platform” recruiting ring components to initiate cytokinetic ring formation at the medial cortex. During ring compaction, anchorage of mid1 C terminus to membranes ensures a precise positioning of the contractile ring.