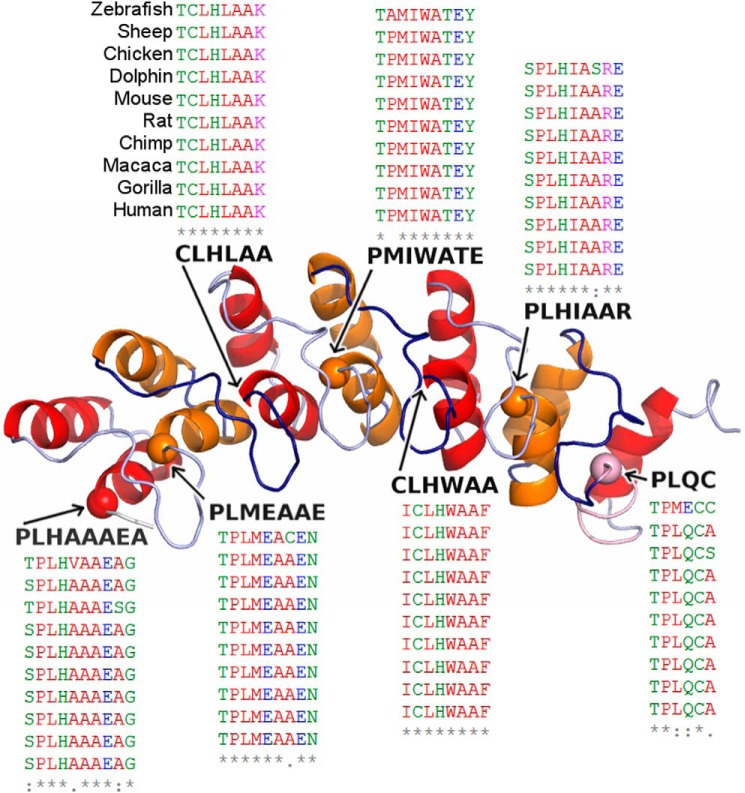
FIGURE 1.
Diagram of the repeated helix-loop-helix domains that characterize the ankyrin repeat of the EHMT1. Our model of EHMT1 is shown with repeats colored alternating between red and orange. Each repeat has an outer and an inner helix. The inner helix always begins with a proline or cysteine and is followed by a conserved TPLX motif. The proline α carbons from these motifs are marked with a sphere, and the sequence of each motif is shown. The p.P809L falls within the third helix and begins the motif within the second ankyrin repeat. To indicate the conservation of TPLX motifs, a section from a multiple sequence alignment of EHMT1 orthologs is shown for each; the species order is shown once for brevity; alignment and corresponding coloring were performed using Clustal Omega (33).