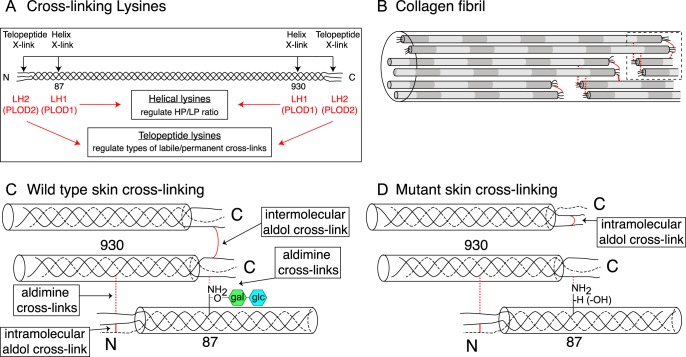
FIGURE 8.
Model speculating how collagen cross-link formation is affected in P3h3−/− and Sc65−/− mouse skin. Under normal conditions, LH1 catalyzes the hydroxylation of helical lysines 87 and 930; and LH2 catalyzes the hydroxylation of the N- and C-telopeptide lysines (A). In the fibril, collagen molecules are spatially arranged such that intermolecular cross-link placement is optimal (B). In WT skin, fully glycosylated Hyl87 preferentially forms an intermolecular aldimine cross-link with a C-telopeptide lysine aldehyde (C). In the P3h3−/− and Sc65−/− mouse tissues the LH1 substrates are under-hydroxylated and subsequently under-glycosylated, which alters collagen cross-linking chemistry (D). From mutant skin the results are consistent with the C-telopeptide lysine aldehydes preferentially forming intramolecular aldol cross-links (as opposed to intermolecular aldols). We predict that under normal conditions the presence of the disaccharide on Hyl87 favors aldimine formation with a single C-telopeptide aldehyde and hinders intramolecular aldol formation with a second α1(I) C-telopeptide from the same molecule as the first one, so favoring intermolecular interactions. The net effect of under-hydroxylated Lys-87 then would be fewer stable aldol intermolecular cross-links within and between fibrils.