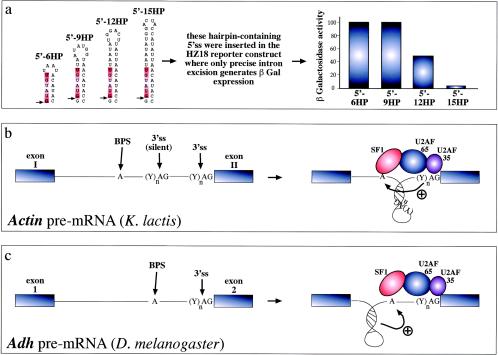
FIG. 3.
RNA structural elements and splicing efficiency. (a) Series of short artificial hairpins (HP) of increasing length containing a 5′ splice site (indicated by an arrow, while the consensus sequence is highlighted in red). These constructs were engineered in the yeast RP51A intron and assayed for functionality in a reporter construct which contains a wild-type intron inserted in the lacZ gene. In this construct, only precise excision of the intron generates β-galactosidase expression. Following transfection in yeast cells, it was determined that longer hairpins have an increasing ability to sequester the donor site and inhibit the early steps of spliceosome assembly (43). Hairpins can also change the relative distances of splicing regulatory elements and thus affect the final outcome. For example, in the yeast actin intron (b) the branch-point sequence (BPS) is located far away from the 3′splice site (3'ss), which is also preceded by a silent 3'ss (silent) that is not normally used by the splicing machinery. The reason for this lies in the proposed folding of the region between the BPS and the correct 3'ss in a hairpin structure. The function of this structure would be twofold: to bring the BPS into working range of the correct 3'ss and to sequester the silent 3'ss, preventing its use by the splicing machinery (33). Finally, hairpin structure formation near the branch point of the Adh gene intron 1 (3c) has been recently proposed to play an active role in splicing through a distinct mechanism. In this case, hairpin formation has been proposed to force the branch point sequence (BPS) into an unpaired conformation that would be better recognized by the splicing machinery (23).