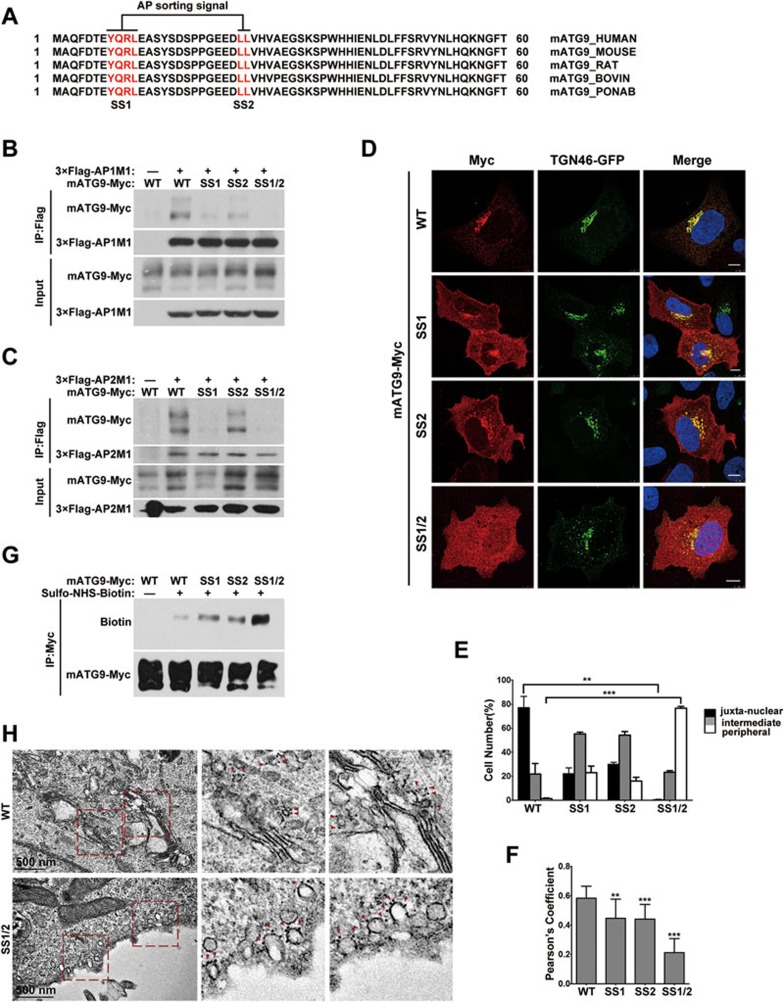
Figure 1.
The mATG9 N-terminus contains two conserved adaptor protein sorting signals. (A) Alignment of mATG9 N-terminus sequences from different mammalian species reveals that two putative AP sorting signals (red color letters) are highly conserved. (B, C) HEK293T cells were transiently co-transfected with wild-type (WT) mATG9-Myc or the indicated mutants and 3×Flag-AP1/2M1. 24 h after transfection, cells were collected for immunoprecipitation with anti-Flag antibody. M1 is the mu subunit of the AP1 and AP2 complexes. (D) U2OS cells were co-transfected with WT mATG9-Myc or the indicated mutants (red) and TGN46-GFP (green). 24 h after transfection, cells were fixed and immunostained with anti-Myc antibody. Cells were counterstained with DAPI (blue). Scale bar, 10 μm. (E) The distribution of mATG9 or the indicated mutants in cells from D was assessed and quantified in a blind fashion (mean ± SEM; n = 100 cells from three independent experiments, **P < 0.01, ***P < 0.001). (F) Pearson's coefficient was determined for the co-localization between mATG9 or the indicated mutants and TGN46 in cells from D. Data were analyzed by ImageJ (means ± SEM; n = 30 cells from three independent experiments, **P < 0.01, ***P < 0.001). (G) HeLa cells were transfected with mATG9-Myc or the indicated mutants for 24 h, and then incubated at 4 °C with ice-cold Sulfo-NHS-Biotin solution for 30 min, rinsed and lysed. Total mATG9 was immunoprecipitated with anti-Myc antibody and the proportion of biotinylated mATG9 was assessed by immunoblotting with anti-Biotin antibody. (H) HeLa cells were transfected with mATG9-Myc or the SS1/2 mutant for 24 h, and then processed for immunogold electron microscopy with anti-Myc antibody. Boxed areas in the left image are shown enlarged on the right. Gold nanoparticles are indicated by red color arrows. Scale bar, 500 nm. See also Supplementary information, Figure S1.