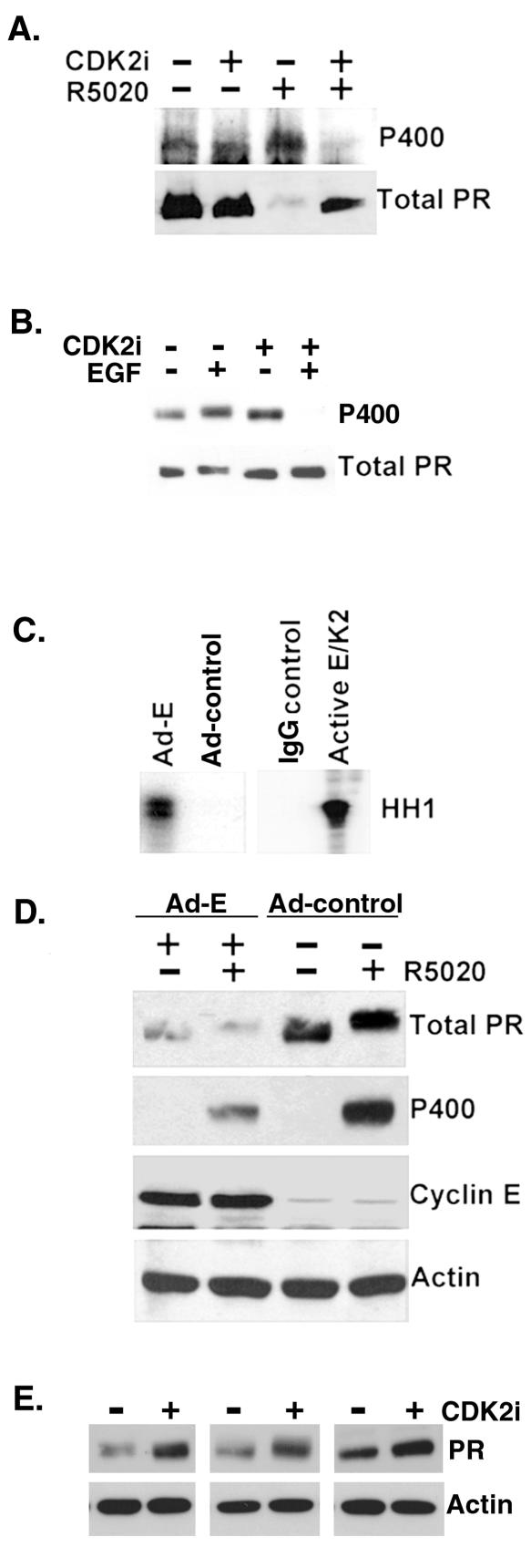
FIG. 2.
CDK2 activity decreases PR protein levels. (A) CDK2 inhibitors block ligand-dependent PR Ser 400 phosphorylation and PR downregulation. T47D-YB cells were preincubated with either vehiclecontrol or a CDK2-specific inhibitor (CDK2 inhibitor II) for 1 h andthen incubated with or without R5020 for an additional 11 h. Cell lysates were blotted for either total PR or phospho-Ser400 PR (P400). (B) CDK2 inhibitors block mitogen-induced phosphorylation of PR Ser400. T47D-YB cells were pretreated with vector control (dimethyl sulfoxide [DMSO]) or the CDK inhibitor roscovatine (700 nM) for 30 min and then treated with R5020 or EGF for 1 h. Cell lysates were blotted for total PR or phospho-Ser400 PR (P400). (C) Adenoviral expression of cyclin E stimulates CDK2 activity. HeLa cells stably expressing PR-B were infected with Ad-cyclin E (Ad-E) or a control adenovirus (Ad-control) for 48 h. CDK2 activity in immunoglobulin G (IgG-control) or CDK2 immune complexes was measured as described above (Fig. 1 legend) with histone H1 (HH1) as a substrate. Purified active cyclin E/CDK2 complexes (1 to 3 ng) were added to the reaction mixture as a positive control for CDK2 activity. (D) Ad-cyclin E induces loss of PR protein. Western blotting for total PR, phospho-Ser400 PR, cyclin E, and β-actin was performed on cell lysates from HeLa cells stably expressing PR-B and transduced with either control adenovirus (Ad-control) or Ad-cyclin E (Ad-E) in the presence and absence of R5020 for 1 h. (E) Inhibition of CDK2 activity stabilizes PR protein. T47D-YB cells were treated with a CDK2-specific inhibitor (CDK2 inhibitor II) or vehicle control (DMSO) for 12 h. Western blotting assays for total PR and actin were performed on cell lysates. Data from three separate experiments are shown.