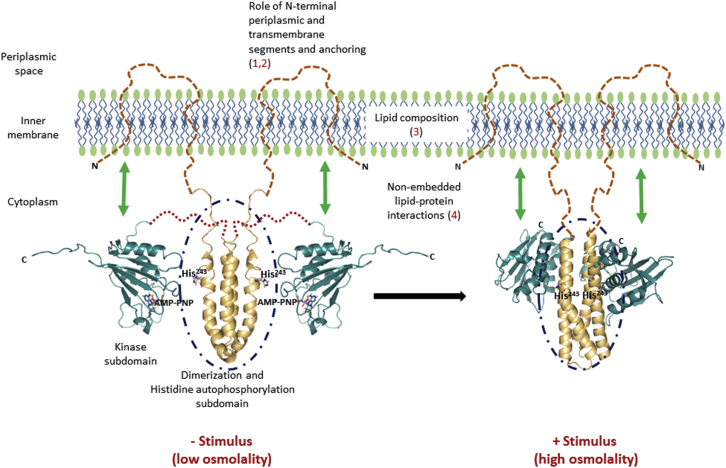
Figure 1.
Membrane lipids alter receptor dynamics and function. The cytoplasmic domain of EnvZ (EnvZc; 180–450) lacking N-terminal TM and periplasmic segments is capable of osmosensing, achieved through stabilization of a locally disordered locus (dark blue dashed circles) spanning the autophosphorylation site His243 (shown as sticks) within the four-helix dimerization subdomain. This establishes this His243-containing locally disordered region as the osmosensing core in EnvZ. However, this raises a question regarding the contribution of 1) the N-terminal 179 residues (the TM and periplasmic domains), 2) membrane anchoring, 3) membrane composition, and 4) potential interactions between the cytoplasmic domain and the membrane to EnvZ dynamics and function. Left: solution NMR structures of the four-helix bundle subdomain (gold, PDB: 1JOY) and the kinase subdomain (teal, PDB: 1BXD) solved separately, denoting the low-osmolality conformation. Large-scale conformational changes are observed in the presence of the osmolality stimulus. Right: x-ray crystal structure of a chimeric construct of the entire EnvZ cytoplasmic domain encompassing both the four-helix bundle (gold) and the kinase subdomains (teal, PDB: 4KP4), denoting the high-osmolality conformation. Structures of the TM and periplasmic regions are currently not available and are depicted as brown dashed lines.