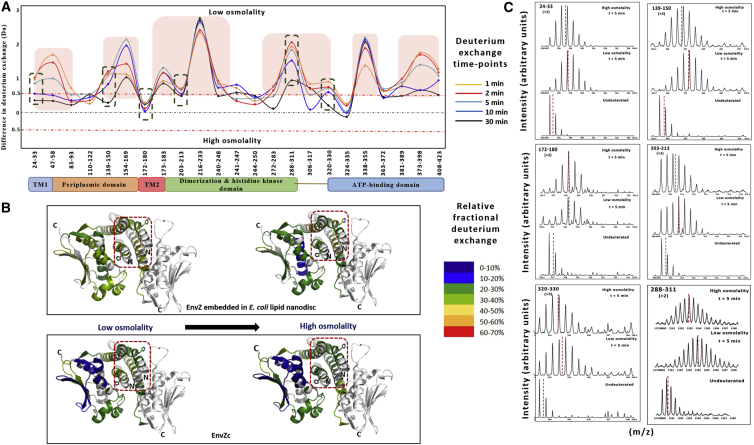
Figure 5.
Osmolyte-induced stabilization of EnvZ embedded in E. coli lipid nanodiscs. (A) A comparison of deuterium exchange between conditions of low and high osmolality in EnvZ embedded in E. coli lipid nanodiscs is demonstrated by a difference plot. A threshold difference of 0.5 Da is considered to be a significant difference and is indicated by the red dashed line on the difference plot. Positive changes denote decreased deuterium exchange, and negative changes denote increased exchange in the presence of high osmolality. EnvZ embedded in E. coli lipid nanodiscs responds to osmolality through stabilization of multiple regions (boxed in red) of the protein, including the TM and periplasmic and kinase domains, besides the dimerization and autophosphorylation domains. (B) The relative fractional deuterium uptake is mapped onto the crystal structure of EnvZc (PDB: 4KP4) under conditions of both low and high osmolality. EnvZc responds to increased osmolality though stabilization of the His243-containing four-helix bundle, but no significant stabilization of the other regions of the protein is observed. In EnvZ embedded in E. coli lipid nanodiscs, osmolyte-induced stabilization is observed in the four-helix bundle along with stabilization of regions within the kinase domain. (C) Electrospray ionization quadrupole time-of-flight mass spectra for different peptides (green dashed boxes in the difference plot) of EnvZ embedded in E. coli lipid nanodiscs are compared under conditions of low and high osmolality.