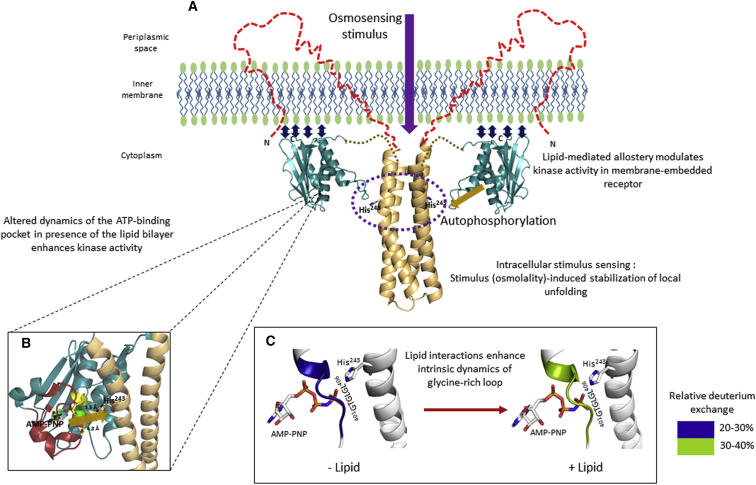
Figure 6.
Peripheral membrane interactions are essential for lipid allostery in EnvZ. (A) The lipid membrane results in a significant increase (∼15-fold) in the in vitro kinase activity of EnvZ. We propose that the increased basal kinase activity of EnvZ is due to lipid allosteric effects mediated by local conformational changes within the kinase subdomain, particularly the ATP-binding pocket. The increase in His243 autophosphorylation due to increased environmental osmolality (denoted by the purple arrow) is independent of membrane effects and is achieved entirely through stabilization of the locally disordered osmosensing core (purple dashed circle) within the four-helix bundle. (B) The ATP-binding pocket (highlighted in red) and the glycine-rich loop (yellow) integrate lipid allosteric effects to enhance catalysis of phosphate group transfer from the kinase subdomain to the His243 residue. The yellow arrow denotes phosphotransfer from the ATP-binding pocket to His243. (C) Lipid interactions enhance the intrinsic dynamics of the glycine-rich loop, as revealed by increased deuterium exchange. We postulate that this enhancement in intrinsic dynamics forms the basis for a faster phosphotransfer rate.