Figure 3. Autophagy controls inflammatory processes.
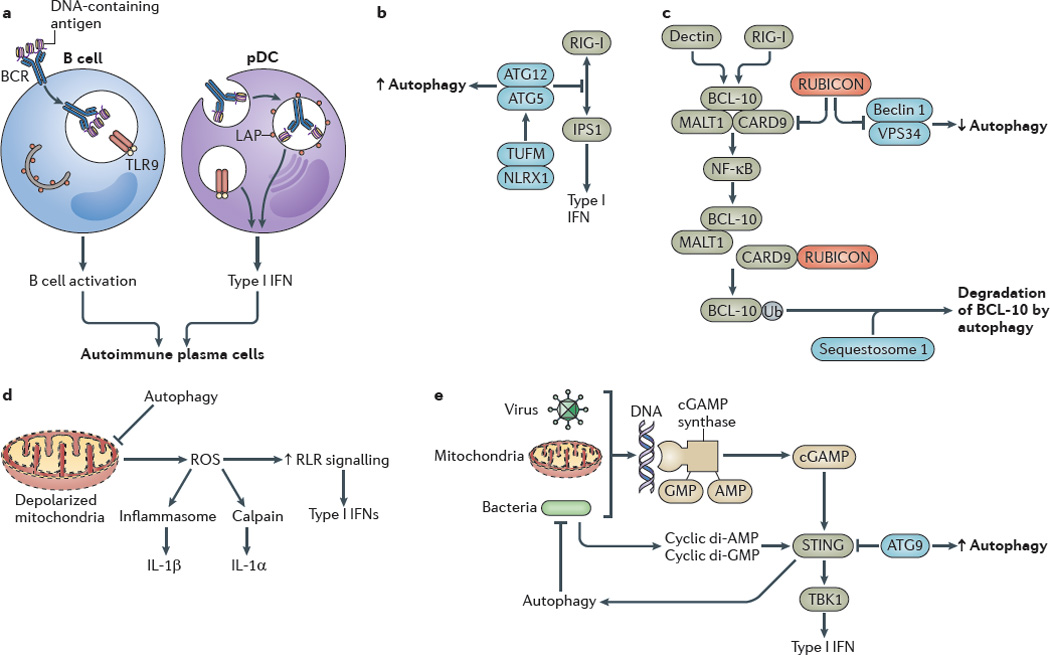
a Autophagy promotes Toll-like receptor 9 (TLR9) signalling in B cells and type I interferon (IFN) production by plasmacytoid dendritic cells (pDCs). b The autophagy protein complex autophagy-related protein 5 (ATG5)–ATG12 inhibits RIG-I-like receptor (RLR) signalling by binding to the caspase recruitment domains of retinoic acid-inducible gene I (RIG-I) and IFNβ _promoter stimulator protein 1 (IPS1), which is the mitochondrial adaptor of RIG-I signalling. The NOD-like receptor X1 (NLRX1)-interacting partner mitochondrial Tu elongation factor (TUFM) associates with the ATG5–ATG12 complex to promote autophagy while inhibiting RLR-dependent type I IFN activation. c Autophagy factors negatively regulate the caspase recruitment domain-containing protein 9 (CARD9)–B cell lymphoma 10 (BCL-10)– mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) complex. RUBICON (run domain beclin 1-interacting and cysteine-rich-containing protein), which is a binding partner and a negative regulator of beclin 1, inhibits CARD9, whereas sequestosome 1 leads to the degradation of BCL-10. d Excessive production of reactive oxygen species (ROS) by depolarized mitochondria that are not cleared by autophagy enhance RLR signalling. e Viral, mitochondrial or bacterial DNA lead to the activation of stimulator of IFN genes protein (STING), probably through cGAMP synthase and cyclic GMP–AMP (cGAMP) production, which increases the type I IFN response. Autophagy removes sources of agonists that stimulate STING, whereas autophagic factors (for example, ATG9) inhibit the activation of STING by affecting its cytoplasmic translocation. Bacterial cyclic dinucleotides (di-AMP and di-GMP) can activate autophagy, thereby functioning as a regulatory loop that amplifies the removal of infectious or endogenous irritants. BCR, B cell receptor; IL, interleukin; LAP, LC3-associated phagocytosis; NK-κB, nuclear factor-κB; TBK1, TANK-binding kinase 1; Ub, ubiquitylation.
