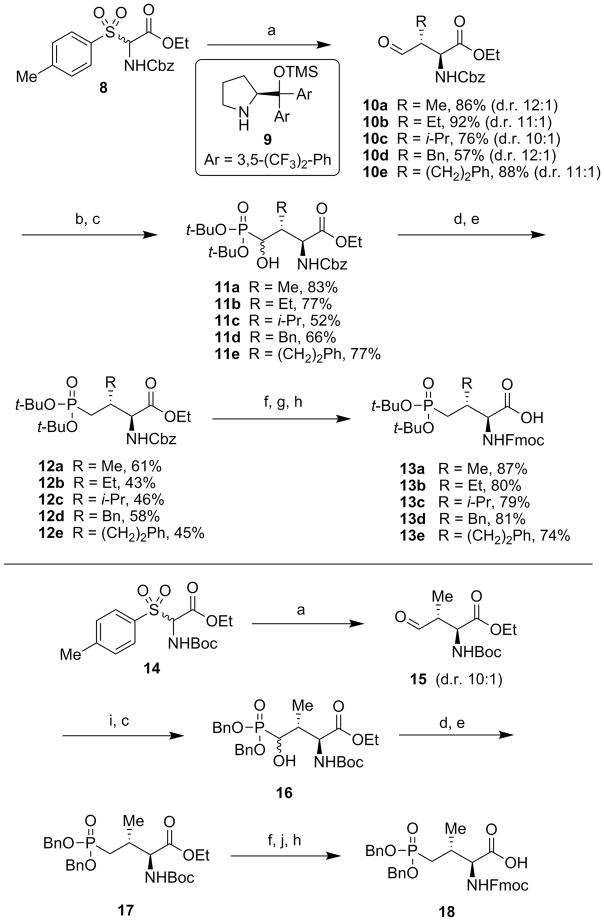
Scheme 1.
Reagents and conditions: a) Aldehyde (2.0 equiv.), KF (5.0 equiv.), 9 (0.1 equiv., 10 mol%), CHCl3, 24 – 96 h, rt (d.r. = diastereomeric ratio); b) di-tert-butyl phosphite (1.5 equiv.), TMS-Cl (1.5 equiv.), TEA (2.0 equiv.), CH2Cl2, 3 – 6 h, rt; c) 20% aq. citric acid (w/v), MeOH, 16 h, rt; d) O-phenyl thiochloroformate (3 equiv.), DIEA (4 equiv.), DMAP (0.2 equiv.), CH2Cl2, rt, 3 – 16 h; e) tributyltin hydride (3.0 equiv.), azoisobutylnitrile (AIBN, 1.0 equiv.), toluene, reflux, 20 min; f) LiOH (3.0 equiv.), THF/H2O (3:1), 16 h; g) 1 atm H2, Pd/C (10% w/w, 0.2 equiv.), MeOH, 3 h, rt; h) Fmoc-OSu (1.5 equiv.), NaHCO3 (5.0 equiv.), THF/H2O (1:1), 16 h, rt; i) di-benzyl phosphite (1.5 equiv.), TMS-Cl (1.5 equiv.), TEA (2.0 equiv.), CH2Cl2, 3 h, rt; j) 20% TFA/CH2Cl2, 2 h, rt.