Figure 7. The proposed role of FILIP1L in inhibiting cancer cell invasion and metastasis through inhibition of WNT signaling.
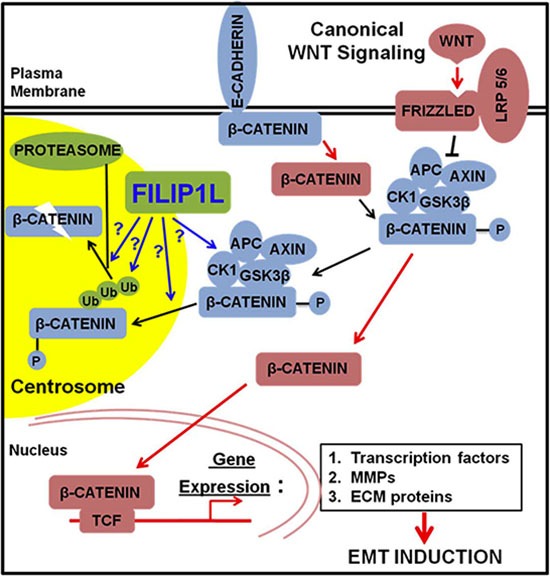
Newly synthesized β-catenin is immobilized by E-cadherin at adherens junctions [10]. β-catenin can be released from the adherens junctions by downregulation of E-cadherin or by the activity of protein kinases. Free excess β-catenin is immediately phosphorylated by the destruction complex and thus marked for subsequent degradation. Canonical WNT signaling blocks the activity of the destruction complex resulting in increased levels of cytolasmic β-catenin, which is translocated to the nucleus. In the nucleus, β-catenin induces the expression of WNT target genes: 1) the transcription factors that repress the expression of E-cadherin; 2) MMPs that cleave E-cadherin as well as extracellular matrix (ECM); 3) the ECM and integrin molecules that favor cell-ECM adhesion. These events lead to induction of EMT. FILIP1L enhances β-catenin degradation in centrosomes possibly through: 1) inactivating the component(s) of β-catenin destruction complex, i.e. inactivating kinase activity of CK1 or GSK3β; 2) facilitating phospho-β-catenin recruitment into centrosomes; 3) facilitating polyubiquitination of phospho-β-catenin; 4) facilitating proteasome-mediated phospho-β-catenin degradation downstream of polyubiquitination. Downstream signaling of active β-catenin (via transcriptional regulation) is thus decreased.
