Figure 2. Loss of Sirt2 enhances KRASG12D-induced lung adenocarcinoma and KRAS acetylation is associated with increased activity in vivo.
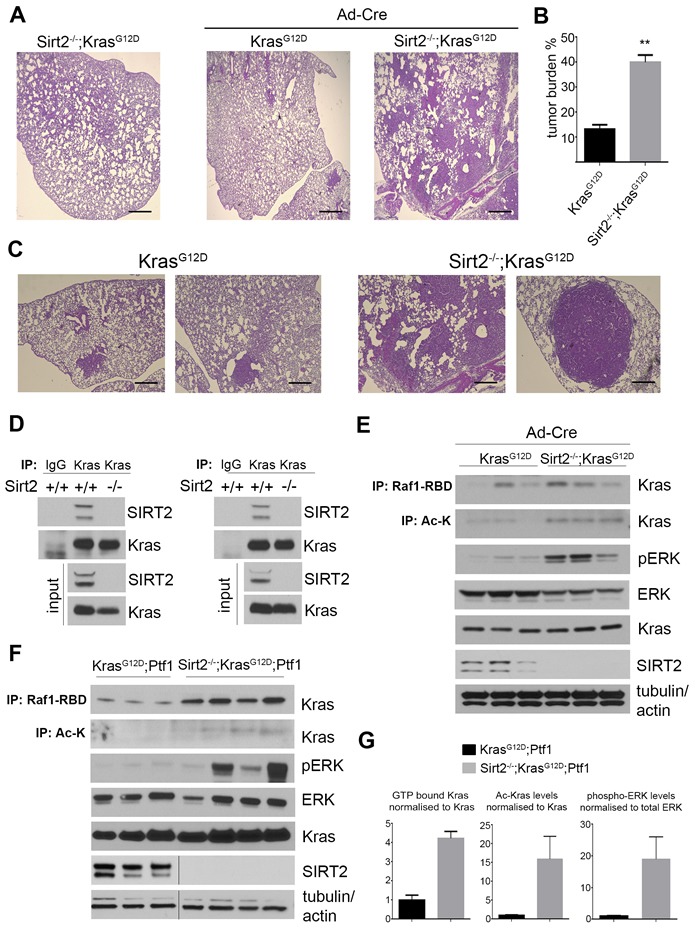
A.-C. The lungs from KrasG12D and Sirt2-/--KrasG12D mice (n = 5 for each genotype), four months after intranasal administration of adenoCRE (Ad-Cre), were harvested, fixed, sectioned, and H&E stained. A. Representative images from lungs (2.5x) of control Sirt2-/--KrasG12D mice (untreated with adenoCRE), and lung tumors developed in KrasG12D and Sirt2-/--KrasG12D mice are shown. Scale bar 50 μM. B. Tumor burden at 4 months in lungs from KrasG12D and Sirt2-/- -KrasG12D mice is presented. Data represent mean ± SEM, **p < 0.01. C. Higher magnification of lung histology in both KrasG12D (left, 10x) and Sirt2-/--KrasG12D (right, 10x) mice is shown. Scale bar 200 μM. D. Endogenous KRAS was immunoprecipitated from lysates of either lung (left) or pancreas (right) tissues. Interaction was confirmed by western blotting using anti-SIRT2 and anti-KRAS antibodies. Endogenous levels of both KRAS and SIRT2 are shown as input. E. The lungs from KrasG12D and Sirt2-/--KrasG12D mice, 2 months after intranasal administration of adenoCRE, were harvested and analyzed for KRAS acetylation and KRAS activity. KRAS acetylation was detected by immunoprecipitation with a pan anti-Ac-K antibody, and KRAS activity was detected by immunoprecipitation with Raf1-RBD and by blotting for pERK. ERK, KRAS, and SIRT2 inputs are shown as controls, and actin and tubulin were used as loading controls. F. The pancreata from KrasG12D-Ptf1 and Sirt2-/--KrasG12D-Ptf1 mice were harvested and analyzed for KRAS activity and KRAS acetylation as described in panel (E). G. Quantification of KRAS activity, KRAS acetylation levels, and phosphorylation levels of ERK from panel (F). Data represent mean ± SEM.
