Figure 7.
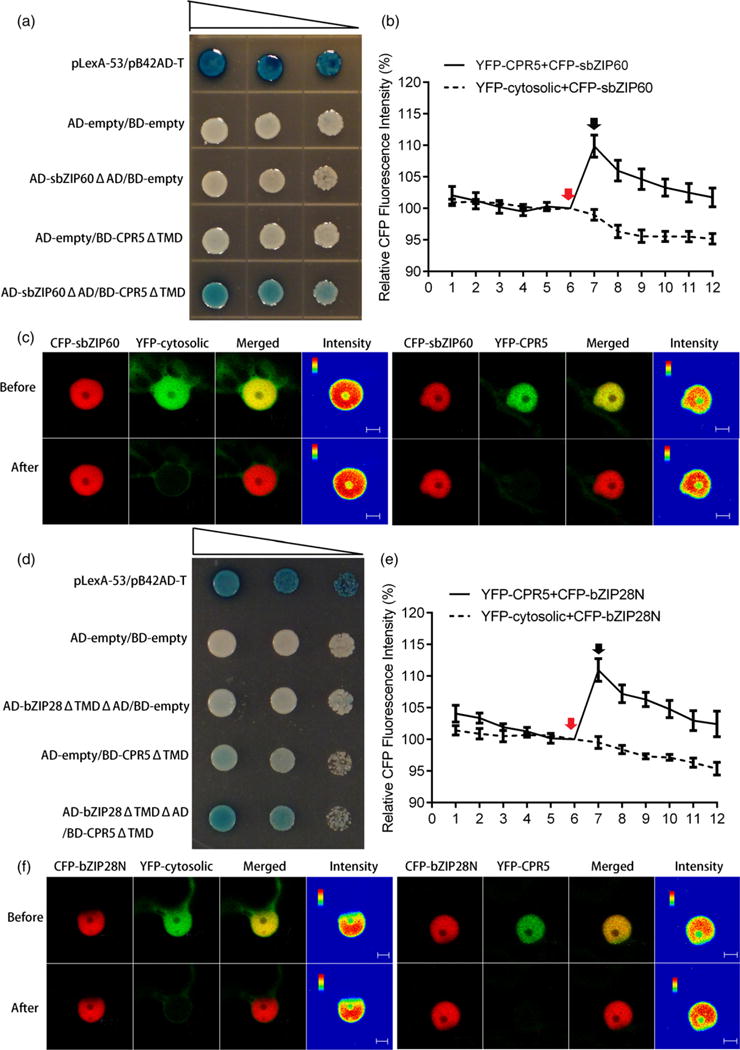
CPR5 interacts with bZIP60 and bZIP28.
(a), (d) Interaction of CPR5 with bZIP60 (a) and bZIP28 (d) in a yeast two-hybrid system. The bait is the N-terminal segment of CPR5 without transmembrane domains (TMD). The N-terminal segments of bZIP transcription factors (TFs) without both transcription activation (AD) and transmembrane domains (TMD) are tested as prey. Yeast co-transformed with the test constructs was grown on SD-Ura-His-Trp plates containing X-Gal. Expression of the lacZ gene (shown by the blue color) was used to indicate of interaction. pLexA-53/pB42AD-T is the positive control. Serial dilutions of transformed cells are shown by narrowing triangles. (b), (e) Quantification of fluorescence resonance energy transfer (FRET) efficiency between YFP–CPR5 and CFP–sbZIP60 (b) or CFP–bZIP28N (e). The percentage of increase in CFP fluorescence after photobleaching YFP (FRET efficiency) was determined. Red arrows indicate pre-bleaching points, and black arrows indicate post-bleaching points. The x-axis indicates the series of images scanned every 10 sec before and after photobleaching. Co-expression of cytosolic YFP and CFP–bZIP was used as negative control. Error bars represent SEM (n = 15). (c), (f) Single-scan confocal images of a representative nucleus taken before and after YFP photobleaching marked by arrows in (b) and (e). For the qualitative detection of FRET, CFP fluorescence images are provided as pseudo-colored intensity maps. Bars = 5 μm.
