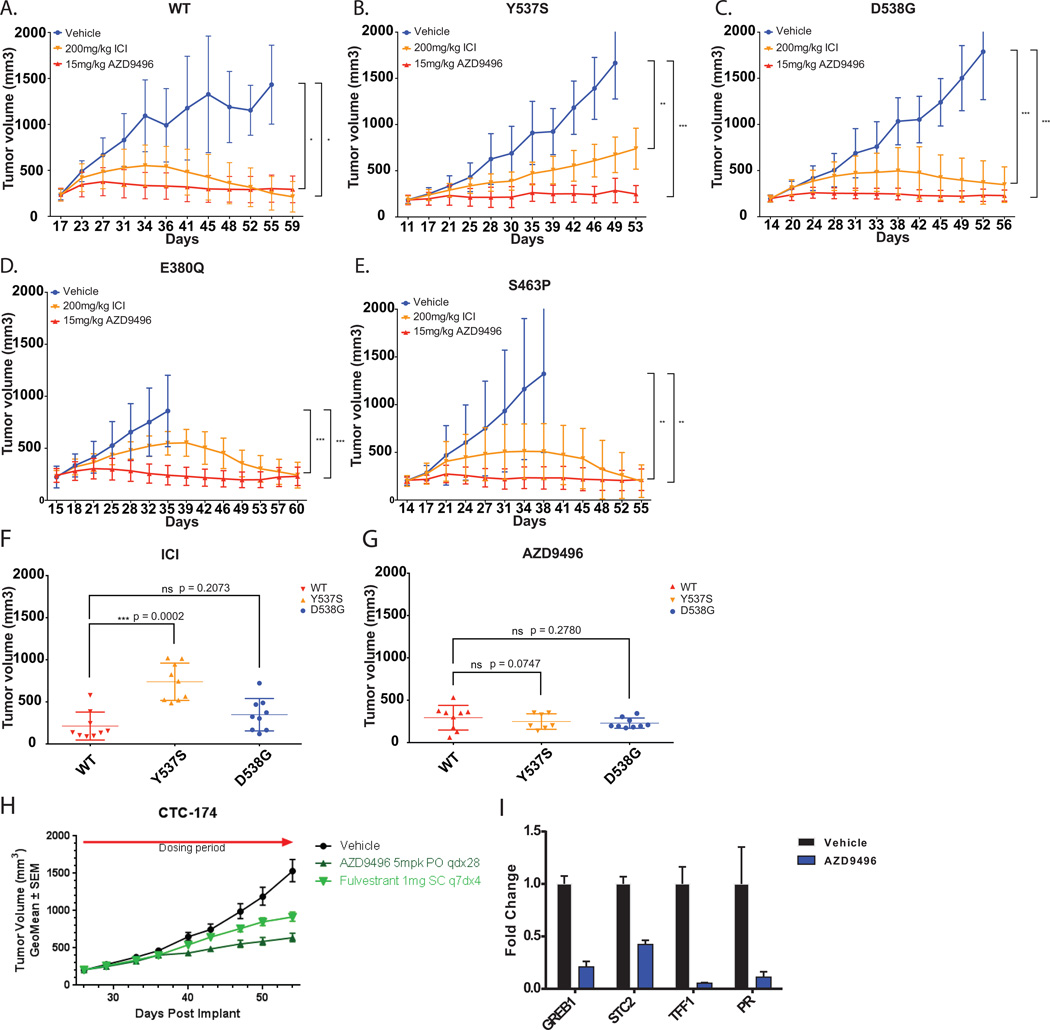
Figure 3. AZD9496 demonstrates superior anti-tumor effects on ER mutant expressing xenografts.
Mice bearing MCF7 inducible HA-ER WT (A), Y537S (B), D538G (C), E380Q (D) or S463P (E) tumors were randomly assigned to treatment groups of either 15 mg/kg of AZD9496, daily orally or 200 mg/kg of Fulvestrant twice weekly, s.c. Tumors treated with AZD9496 showed greater growth inhibition as compared to those treated with Fulvestrant. The result was presented as average tumor volume measured for each group ± SD (n = 10 mice/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (F–G) Scatter plots of the volumes of tumors expressing WT, Y537S or D538G treated with either fulvestrant (ICI) or AZD9496 taken at the end of the xenograft studies shown in A–C. T-tests comparing the volumes of mutant tumors to that of the WT indicated significant resistance of Y537S mutant tumors to fulvestrant treatment. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (H) Patient-derived xenograft (PDX), CTC-714, with D538G mutation, showed greater growth arrest with 5mg/kg of AZD9496. The result was presented as average tumor volume measured for each group ± SEM. (I) Quantitative PCR detection of various ER target genes of AZD9496 treated CTC-714, the D538G PDX model, showed significant reduction in the transcript levels of GREB1, STC2, TFF1 and PGR when compared to the vehicle, indicating inhibition of ER signaling pathway by AZD9496. Graphs were plotted with the mean ± SD of three technical replicates.