Figure 2.
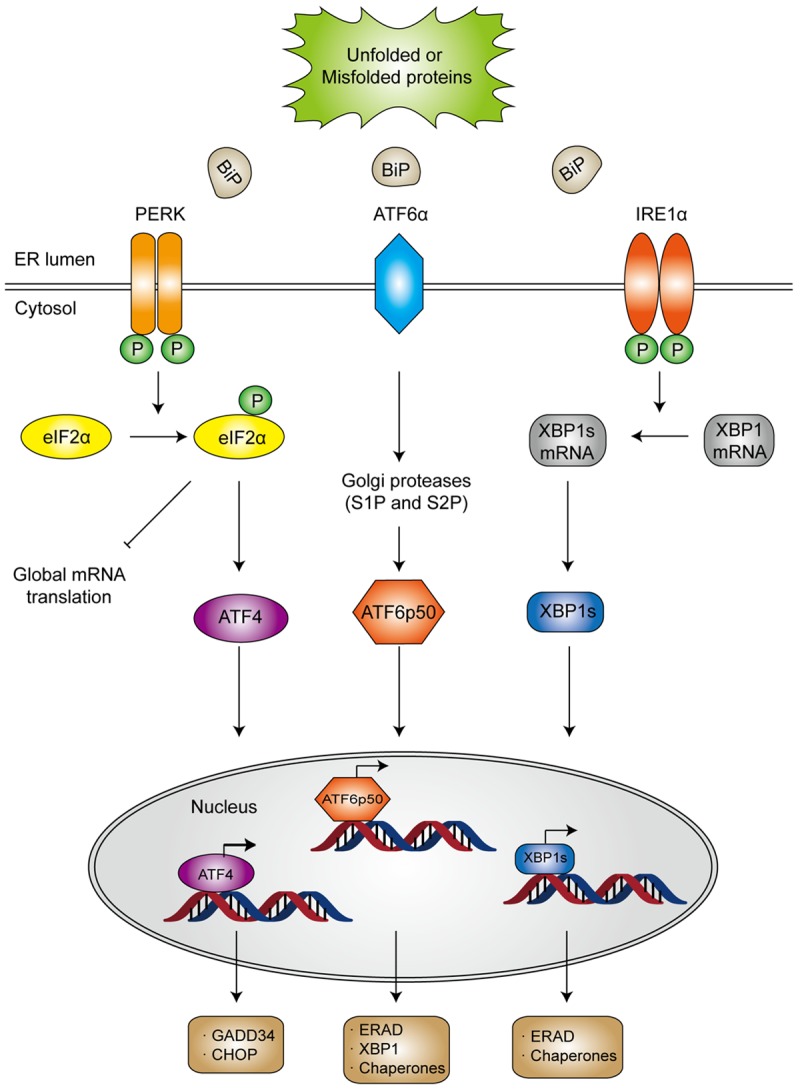
Schematic illustration of ER stress and the activation of three UPR pathways. Under stressed condition, BiP dissociates from the ER stress sensors owing to aggregation of unfold or misfolded proteins in ER lumen, which releases the stress sensors to initiate downstream signaling. Activated PERK undergoes autophosphorylation and dimerization and subsequently inhibits ribosome assembly by phosphorylating the α-subunit of eukaryotic translational initiation factor 2 (eIF2α). Once becomes phosphorylated, eIF2α not only suppresses protein translation, but also upregulates the expression of activating transcription factor 4 (ATF4), which would induce the transcription of protective genes DNA damage-inducible protein 34 (GADD34) as well as the pro-apoptotic gene encoding C/EBP homologous protein (CHOP). In general, CHOP is often produced in the terminal unfolded protein response (UPR) to induce apoptosis. After dissociating from BiP, ATF6α translocates to the Golgi apparatus, where it is cleaved by site 1 protease (S1P) and S2P into an NH2 terminal domain and a cytosolic fragment (ATF6p50). ATF6p50 is then transported into the nucleus and activates the transcription of several ER proteins such as X-box binding protein 1 (XBP1), calreticulin, calnexin, disulfide isomerase and CHOP. Upon activation, IRE-1α dimerizes and cleaves XBP1 into its spliced form, which then acts as a transcription factor of many stress proteins to enlarge the protein-folding capacity of ER, and to induce the expression of ER associated degradation (ERAD)-related proteins such as ER degradation enhancing α-mannosidase-like protein (EDEM).
