Abstract
The World Health Organization (WHO) 2017 Classification of Head and Neck Tumors (“Blue Book”) will now include a new chapter on tumors and tumor-like lesions of the neck and lymph nodes, which was not included in the previous edition. Tumors and tumor-like lesions, including a variety of cysts and metastases, can arise in any component in the neck, including soft tissue, lymph nodes, and developmental remnants. The pathology and clinical features of metastatic carcinoma of unknown primary in the head and neck has changed dramatically in the last several years. Many of these tumors which were previously diagnosed as unknown primary are now identified as oropharyngeal and nasopharyngeal carcinomas related to human papillomavirus (HPV), less commonly to Epstein-Barr virus (EBV) and occasionally even to Merkel cell polyomavirus. Many unusual features can arise in these metastases, such as undifferentiated morphology, extensive cystic change with central degeneration, gland formation, and even ciliated cells. Rarely, carcinoma in the neck can arise in association with a heterotopic tissue, primarily thyroid or salivary gland tissue. Tumor-like lesions include branchial cleft cysts, thyroglossal duct cyst, dermoid and teratoid cyst, and ranula. Pathologists should be familiar with the diagnostic features and clinicopathologic corrections of these neck lesions in order to correctly diagnosis them and to provide for proper clinical management. This article will briefly describe the pathologic and clinical features of these entities as they are covered in the new 2017 Blue Book.
Keywords: World Health Organization, Neck, Metastatic squamous cell carcinoma, Tumor-like, Cysts, Unknown primary, Merkel cell
Introduction
Periodic updates of the WHO/IARC Classification of Tumours reference books (i.e., “Blue Books”) permit intermittent additions of new entities and refinements of tumor classification based on the ever advancing state of science.
One obvious absence has been tumors and tumor-like lesions of the neck and lymph nodes. While most of these lesions are related to mucosal-based tumors metastasizing to neck lymph nodes, there are a number of lesions arising specifically from the neck and its various structures.
Further, since a significant fraction of carcinomas in modern practice (particularly those related to viruses) will present as metastases in the neck lymph nodes either with a clinically occult or completely unidentifiable primary lesions [1], a thorough classification, discussion, and proposal of taxonomy for neck tumors is thus important for a complete head and neck Blue Book. The 2017 edition will now include such a chapter.
Since the non-hematopoietic neck lesions are new to the Blue Book, this review article will briefly discuss all of them, focusing on novel aspects, diagnostic features, potential pitfalls, and clinicopathologic correlations that are critical to diagnosis, reporting, and clinical management of these patients.
Tumors of Unknown Origin
Metastatic carcinomas of unknown primary in the head and neck are becoming more frequent [2].
They have had a strong impact on contemporary clinical pathology practice and are something that all pathologists should be thoroughly familiar with. Interestingly, a large fraction of these tumors are related to oncogenic viruses, primarily human papillomavirus (HPV), but also Epstein-Barr virus (EBV) and occasionally even Merkel cell polyomavirus [1–4].
Metastatic Squamous Cell Carcinoma of Unknown Primary
One of the most important changes in contemporary head and neck pathology practice has come from the HPV oropharyngeal squamous cell carcinoma (OPSCC) epidemic [5]. Rates of these cancers have increased dramatically, particularly in the US and other Western countries, in the past several decades [5], as much as 5% per year.
Tumors are characterized by transcriptionally-active high risk HPV [6], p16 overexpression, high rates of nodal metastases despite small primary tumors, and greatly improved prognosis relative to conventional head and neck SCC [7, 8]. The impact on neck lesions has been dramatic.
As many as 90% of patients with HPV-positive OPSCC have neck lymph node metastases at presentation [7, 8] and approximately 50% will present with neck, rather than primary tumor, symptoms [9]. Since the primary tumors are often small, arise in the reticulated tonsillar crypt epithelium deep to the surface, and engender little stromal reaction, they frequently present as neck nodal metastases without a clinically obvious primary lesion [9]. Approximately 80% of patients presenting with metastatic SCC of unknown primary in the neck are related to transcriptionally-active high risk HPV. After thorough clinical work up, most of the primary tumors that are found are found in the oropharynx. Of the remainder, it is approximately 35–40% [1]. Several peculiar pathologic features can be seen. First, neck nodal metastases related to HPV-positive OPSCC are frequently large with pushing borders and expansion of the lymph nodes rather than extensive infiltration of the surrounding tissue [10]. In addition, they are very frequently cystic and/or necrotic [11]. In fact, exquisitely cystic lymph node metastases in the neck are highly correlated with HPV-positive OPSCC primaries. This brings up the differential diagnosis for cystic neck lesions, which are quite common (Table 1). Most of these are specifically discussed in the new Blue Book. The morphology of metastatic HPV-positive OPSCC is most commonly nonkeratinizing [12, 13] (Fig. 1a, b). There is often maturing squamous differentiation, but it is usually limited in extent. HPV-positive OPSCC and unknown primary tumors can be keratinizing-type or almost any of the other specific SCC variants. Sometimes the cystic lesions are full of acellular keratin debris. Fine needle aspiration specimens, particularly if they sample the cyst contents without focusing specifically on sampling the solid walls of the lesion, can be false negative [14], can have limited cellularity affecting p16 immunohistochemistry [14–16], and/or can have morphologic features suggestive of benign cysts such as branchial cleft cysts or abscesses. Further complicating matters is that metastatic HPV-positive OPSCC can develop true gland formation (“adenosquamous carcinoma”) and tumor cells lining the glandular and cystic foci can have cilia. This has been termed “ciliated nonkeratinizing adenosquamous carcinoma [17, 18].” Cilia and bland cytologic features in cystic neck lesions, thus, are not necessarily indicative of a benign process. Metastatic HPV-negative SCC usually, but not always, has the keratinizing morphology typical of conventional head and neck SCC. Primary sites, when found, include the pyriform sinus, oral cavity, and sometimes skin or non-head and neck sites [4, 19]. One other lesion must also be considered in the differential diagnosis. Patients with EBV-related undifferentiated nasopharyngeal carcinoma can also present with lymph node metastases without a clinically apparent primary (Fig. 1c, d) [3, 4]. These are often in levels II–IV, but also frequently occur in level V. When one encounters metastatic SCC in a core biopsy, excisional biopsy, or neck dissection specimen, the morphology should be examined closely and p16 immunohistochemistry performed. The combination of nonkeratinizing morphology, p16 positivity, and a jugular chain lymph node is sufficient evidence to suggest in oropharyngeal primary in the report. For nonkeratinizing or undifferentiated carcinomas that are p16 negative, EBV in situ hybridization (EBER) should be performed. The combination of undifferentiated morphology and EBV positivity is sufficient evidence to strongly suggest an occult nasopharyngeal primary in the report. It should also be mentioned that the newly published American Joint Cancer Commission (AJCC) and Union for International Cancer Control (UICC) actually provide an algorithm for workup of SCC of unknown primary that includes HPV and EBV testing. Tumors positive for the viruses are staged according to the schema for oropharynx and nasopharynx, respectively. For lymph node metastases that are keratinizing SCC or are located outside of the jugular chain, if the p16 comes back diffusely positive, then HPV-specific testing must be performed as a specificity check given the increased rates of nonspecific p16 positivity in other settings, particularly in skin [20] and lung SCC [21].
Table 1.
Differential diagnosis of cystic neck lesions
| Metastatic squamous cell carcinoma |
| Branchial cleft cyst |
| Thyroglossal duct cyst |
| Metastatic papillary thyroid carcinoma |
| Heterotopia-associated carcinoma |
| Dermoid and/or teratoid cyst |
| Lymphangioma/hemangioma |
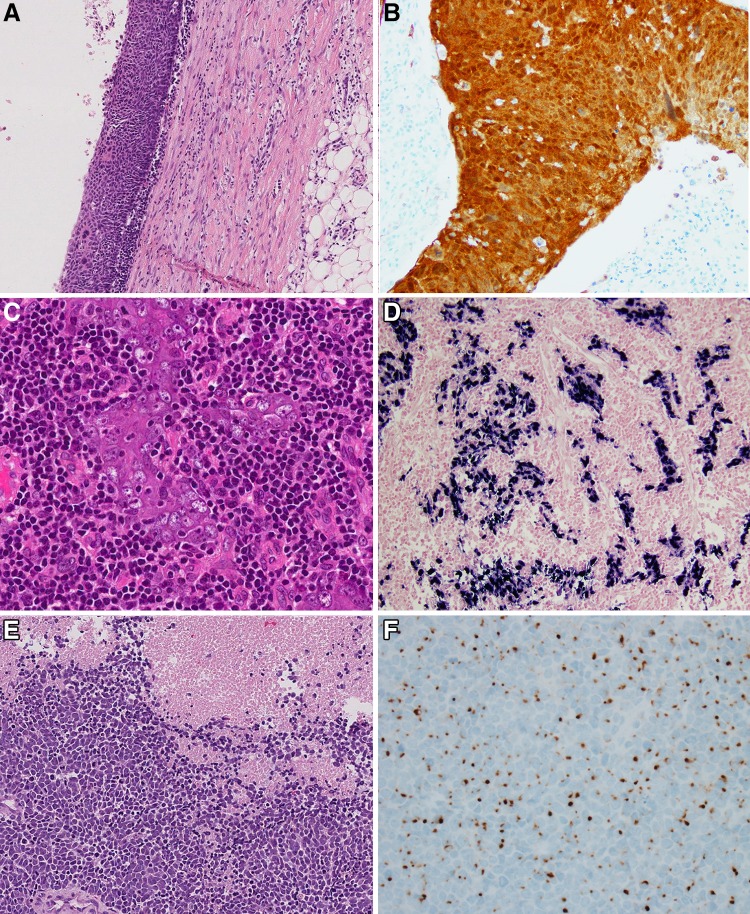
Fig. 1.
Metastatic carcinoma of unknown primary. a HPV-positive oropharyngeal squamous cell carcinoma with cystic change and lining nonkeratinizing epithelium (×4 magnification); b HPV positive squamous cell carcinoma showing diffuse p16 positivity (×10 magnification); c EBV-related metastatic nonkeratinizing undifferentiated nasopharyngeal carcinoma (×10 magnification); d EBV (EBER) positivity by in situ hybridization (×10 magnification); e Metastatic Merkel cell carcinoma (×20 magnification); f Merkel cell carcinoma showing cytokeratin 20 positivity with dot-like, perinuclear accentuation (×20 magnification)
Metastatic Merkel Cell Carcinoma of Unknown Primary/Neck Lymph Nodes
The new fourth edition Blue Book specifically includes a section on Merkel cell carcinoma (MCC) in the neck. While MCC in the neck lymph nodes almost certainly represents metastases from occult or regressed primary skin lesions, the literature suggests that there may be a “primary nodal” form of MCC. Morphologically, these tumors are the same as those seen in the skin. A characteristic morphologic appearance, seen only in a subset of MCCs, is sheets of overlapping, perfectly round nuclei with blast-like chromatin and mitotic activity (Fig. 1e) [22]. More often, however, they have the appearance similar to lung small cell carcinoma with angulated, tapered nuclei, speckled chromatin, and nuclear molding. MCC is characteristically positive for cytokeratin 20 with a dot-like pattern (Fig. 1f) [23]. Merkel cell polyomavirus can be detected in one-third of metastatic MCC of unknown primary [24], but in most cases, direct testing for the virus in pathology specimens is not necessary. In routine clinical practice, diagnostic keys are to: (1) recognize a neuroendocrine nature of the tumor, (2) evaluate for immunohistochemistry for neuroendocrine markers, cytokeratin 20, and thyroid transcription factor 1, and (3) for tumors confirmed as cytokeratin 20 positive, mentioning in the report to evaluate for an occult skin or primary salivary gland tumor. Interestingly, the prognosis for nodal MCC appears to actually be better than for MCC of known primary [25].
Heterotopia-Associated Carcinoma
Carcinoma arising from ectopic tissue in the neck (heterotopia-associated carcinoma) is rare, accounting for <1% of head and neck carcinomas. The majority of carcinomas arise from salivary gland or thyroid tissue [26–28]. Heterotopia-associated thyroid carcinoma may arise in ectopic thyroid tissue (lingual, tracheal, midline, or lateral neck) or branchial cleft or thyroglossal duct cysts. Salivary gland type carcinomas usually seen in the peri-parotid lymph nodes or along the anterolateral border of the sternocleidomastoid muscle [29]. Histologically, papillary thyroid carcinoma is the most common type thyroid carcinoma seen in heterotopic sites. Other types include: follicular carcinoma, Hurthle cell carcinoma, anaplastic thyroid carcinoma, and medullary carcinoma [28]. About 80% of tumors arising in heterotopic salivary gland are benign such as Warthin’s tumor and pleomorphic adenoma. Mucoepidermoid carcinoma is the most common carcinoma. It is important to differentiate heterotopia-associated carcinoma from nodal metastasis from undetected thyroid or salivary gland primary. The absence of other primaries and the presence of the non-neoplastic heterotopic tissue help to differentiate heterotopia-associated carcinoma from nodal metastasis. In view of their relative rarity, clinical outcome and management of heterotopia-associated carcinomas are not well delineated. The reported follow up is highly variable ranging from 1 month to 17 years. However, the most important reported prognostic factors seem to be tumor size, stage, type, and histologic grade [27, 28].
Branchial Cleft Cyst
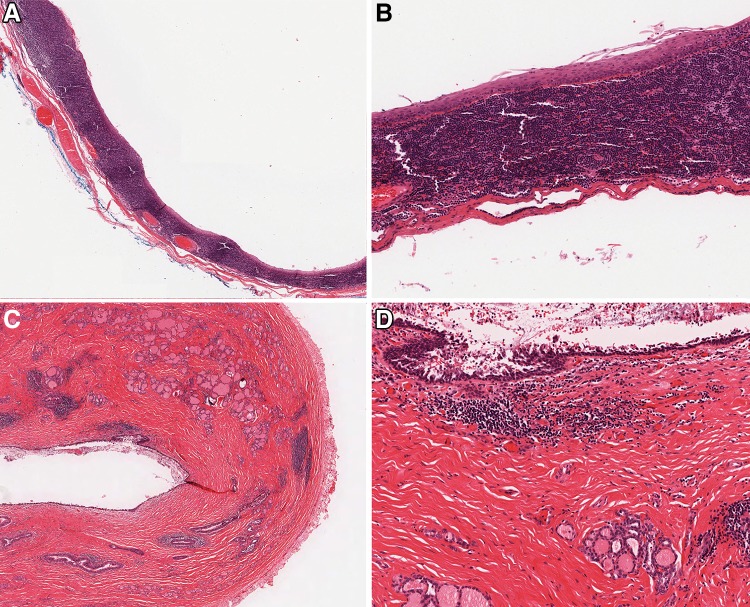
Branchial cleft cysts are congenital developmental cervical cysts that are thought to be derived from remnants of the second branchial apparatus [30–32]. These occur in the lateral neck near the mandibular angle, accounting for 90% of lateral, and 20% of cervical cysts, overall [33, 34]. Patients usually present with painless swelling in the neck. There are bimodal age peaks identified at 20–40 years (accounting for 75%) and at <5 years (accounting for 20%) [33, 34]. Branchial cleft cyst is usually unilocular and may measure up to 10 cm. The wall of the cyst is smooth containing mucoid or watery material. Histologically, the cysts are commonly lined by non-stratified squamous epithelium and less commonly by ciliated respiratory epithelium with occasional goblet cells (Fig. 2a, b). The cyst wall has organized lymphoid tissue with germinal centers. Branchial cleft cysts are treated by complete surgical excision. Although SCC has been reported in branchial cleft cysts, malignancy arising in branchial cleft cysts is extremely controversial. The diagnosis of cystic metastasis should always be considered in this setting, especially in patients older than 40 years [35]. Squamous maturation, organized and orderly arrangement of the epithelium, the lack of significant cytologic atypia and mitoses, and the intimate admixture of the lining epithelium with the stroma help to differentiate branchial cleft cyst from metastatic SCC [36]. Of note, p16 is expressed in >50% of branchial cleft cysts which somewhat limits its utility in separating branchial cleft cyst from metastatic HPV-positive SCC. However, the pattern can be extremely helpful. P16 is typically focal and limited to the superficial epithelium in branchial cleft cysts whereas in SCC, the staining is diffuse [36].
Fig. 2.
Branchial cleft and thyroglossal duct cysts. a Low power view of a branchial cleft cyst showing a thin lining epithelium and dense associated lymphoid tissue with germinal centers (×2.5 magnification); b Branchial cleft cyst lining which is thin, squamous, and has no atypia, extensive maturation, and minimal keratinization (×10 magnification); c Low power view of a thyroglossal duct cyst showing a cystic structure with an attenuated lining and adjacent smaller cystic foci mixed with thyroid follicles (×2.5 magnification); d Thyroglossal duct cyst showing an attenuated and partially denuded respiratory-epithelial lined cyst and scattered bland thyroid follicles in the dense surrounding connective tissue (×10 magnification)
Thyroglossal Duct Cyst
Thyroglossal duct (TGD) cysts are the persistent cystic dilation of the thyroglossal duct and the most common congenital mass in the neck. The majority of cases occur in the midline of the neck or within 2 cm of it at the level of hyoid bone [37, 38]. Endodermal tissue at the base of tongue descends in the midline neck to form the thyroid gland. Most patients are asymptomatic but the cyst may present as fistula, draining sinus, or recurrent neck swelling that moves with swallowing [39]. Grossly, TGD cysts are usually small (<2 cm), ranging from 0.5 to 10 cm in size, showing smooth lining, [40, 41] and containing thin and mucoid material. Solid excrescences should raise the suspicion for malignancy [39]. Histologically, TGD cyst is typically lined by respiratory or squamous epithelium and may show mucous gland in the wall (Fig. 2c, d). The presence of thyroid tissue in the cyst’s wall varies and can be found in >60% of the cases. Subepithelial lymphoid tissue resembling branchial cleft cyst can be seen in some cases. Severe inflammation may dislocate the cyst and obliterate the cyst lining. In such situations where the epithelial lining and thyroid follicles are difficult to find due to inflammation and fibrosis, a diagnosis of developmental cyst may be an option. Recurrences can occur after inadequate excision [42]. Malignancy, most often papillary thyroid carcinoma, may occur in TGD cyst. Extended surgery with en block resection of the cyst, the middle third of the hyoid bone (Sistrunk procedure), and the suprahyoid tract is the treatment of choice. The prognosis of TGD cyst even if associated with papillary thyroid carcinoma is usually excellent [43, 44].
Ranula
Ranula, also called mucocele, mucus extravasation, and retention cyst, is a rare lesion characterized by an abnormal pooling of mucus within an intraoral cystic cavity. The lesion is usually associated with the sublingual gland [45]. The most common etiology is the trauma to an excretory duct. The lesions are usually unilateral and unifocal, but may be bilateral or multiple [46]. Clinically, there are two types: simple and plunging (deep) [46, 47]. Simple ranula presents as a painless mass in the oral cavity floor whereas plunging ranula presents as an asymptomatic neck mass. Simple ranula usually occurs in the lateral floor of the mouth in association with the excretory duct of the sublingual gland [46]. In plunging ranula, extravasated mucin dissects through the muscle of the floor of the mouth into the neck [47]. Ranula is typically a blue, fluctuant, painless mass that can reach several centimeters in size [29]. Histologically, a simple ranula is a cyst that contains mucin and may be focally lined by epithelium, which can be squamous, cuboidal, or columnar. A plunging ranula is considered as a pseudocyst that lacks a lining epithelium and shows a pool of mucin surrounded by fibrous tissue and inflammatory cells (Fig. 3). The lesions are treated by complete excision, including removal of the traumatized salivary duct but inadequate excision can result in recurrence [47].
Fig. 3.
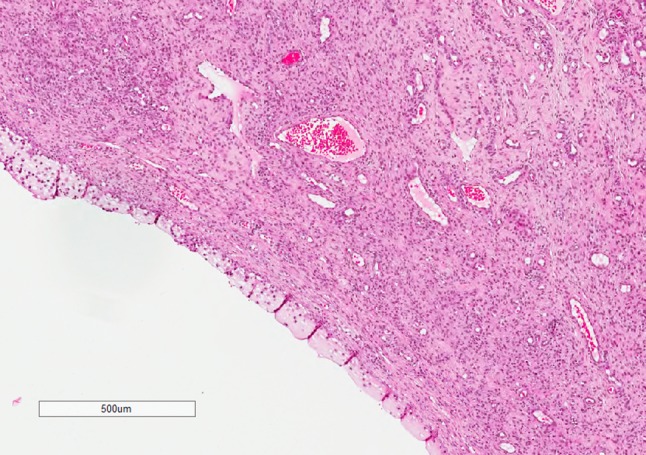
Plunging ranula (×10 magnification). Unilocular cyst filled with mucin and surrounded by inflamed fibrous wall
Dermoid and Teratoid Cysts
Dermoid cyst is a cyst that contains both ectoderm and mesoderm derived tissues. The term teratoid cyst is typically used when additional endodermal derivatives are present. The cyst occurs in the head and neck in 7% of the cases [48]. The majority of the cysts are subcutaneous and recognized in children younger than 5 years old [49]. The most common sites include midline neck or nose, nasolabial fold, and lateral third of the eyebrow (along the embryological closure lines) [50]. Grossly, the cyst has yellowish-white keratinous material and might reach 12 cm in size. Histologically, dermoid cyst is lined by stratified squamous epithelium associated with hair follicles and sebaceous glands. The absence of cutaneous adnexal structures is indicative of epidermoid cyst. In contrast to teratoid cyst, dermoid cyst lacks endodermal derivatives (e.g. gastrointestinal or respiratory mucosa or smooth muscle).
Conclusion
In summary, tumor and tumor-like lesions in the neck and lymph nodes are now covered by a specific chapter in the 2017 WHO Blue Book. They include a variety of benign and malignant lesions and can have a variety of unusual and confusing clinical and histologic features. The diagnosis, particularly for cystic lesions, varies from benign, completely innocuous developmental lesions to carcinomas, some of which are aggressive and others indicative of established metastatic disease. The implications are major. When evaluating a neck lesion/tumor, understanding the anatomy, diagnostic features, and clinical features is critical to diagnosis, and the new Blue Book now provides an excellent reference for pathologists to utilize.
Compliance with Ethical Standards
Conflict of interest
Neither author has any conflicts of interest to disclose.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Special Issue: World Health Organization Classification Update
References
- 1.Boscolo-Rizzo P, Schroeder L, Romeo S, Pawlita M. The prevalence of human papillomavirus in squamous cell carcinoma of unknown primary site metastatic to neck lymph nodes: a systematic review. Clin Exp Metastasis. 2015;32(8):835–845. doi: 10.1007/s10585-015-9744-z. [DOI] [PubMed] [Google Scholar]
- 2.Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsague X, Laporte L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 3.Bussu F, Sali M, Gallus R, Petrone G, Autorino R, Santangelo R, et al. HPV and EBV infections in neck metastases from occult primary squamous cell carcinoma: another virus-related neoplastic disease in the head and neck region. Ann Surg Oncol. 2015;22(Suppl 3):S979–S984. doi: 10.1245/s10434-015-4808-5. [DOI] [PubMed] [Google Scholar]
- 4.Tong CC, Luk MY, Chow SM, Ngan KC, Lau WH. Cervical nodal metastases from occult primary: undifferentiated carcinoma versus squamous cell carcinoma. Head Neck. 2002;24(4):361–369. doi: 10.1002/hed.10054. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36(12):1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35(9):1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 9.McIlwain WR, Sood AJ, Nguyen SA, Day TA. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(5):441–447. doi: 10.1001/jamaoto.2014.141. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 12.Gondim DD, Haynes W, Wang X, Chernock RD, El-Mofty SK, Lewis JS Jr. Histologic typing in oropharyngeal squamous cell carcinoma: a 4-year prospective practice study with p16 and high-risk HPV mRNA testing correlation. Am J Surg Pathol. 2016. [DOI] [PubMed]
- 13.Chernock RD. Morphologic features of conventional squamous cell carcinoma of the oropharynx: ‘keratinizing’ and ‘nonkeratinizing’ histologic types as the basis for a consistent classification system. Head Neck Pathol. 2012;6(Suppl 1):S41–S47. doi: 10.1007/s12105-012-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalaly JB, Lewis JS, Jr, Collins BT, Wu X, Ma XJ, Luo Y, et al. Correlation of p16 immunohistochemistry in FNA biopsies with corresponding tissue specimens in HPV-related squamous cell carcinomas of the oropharynx. Cancer cytopathology. 2015;123(12):723–731. doi: 10.1002/cncy.21600. [DOI] [PubMed] [Google Scholar]
- 15.Holmes BJ, Maleki Z, Westra WH. The fidelity of p16 staining as a surrogate marker of human papillomavirus status in fine-needle aspirates and core biopsies of neck node metastases: implications for HPV testing protocols. Acta Cytol. 2015;59(1):97–103. doi: 10.1159/000375148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Ghossein R, Lane J, Lin O, Katabi N. The utility of p16 immunostaining in fine needle aspiration in p16-positive head and neck squamous cell carcinoma. Hum Pathol. 2016;54:193–200. doi: 10.1016/j.humpath.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JA, Westra WH. Ciliated HPV-related carcinoma: a well-differentiated form of head and neck carcinoma that can be mistaken for a benign cyst. Am J Surg Pathol. 2015;39(11):1591–1595. doi: 10.1097/PAS.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radkay-Gonzalez L, Faquin W, McHugh JB, Lewis JS, Jr, Tuluc M, Seethala RR. Ciliated adenosquamous carcinoma: expanding the phenotypic diversity of human papillomavirus-associated tumors. Head Neck Pathol. 2016;10(2):167–175. doi: 10.1007/s12105-015-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cianchetti M, Mancuso AA, Amdur RJ, Werning JW, Kirwan J, Morris CG, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119(12):2348–2354. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 20.McDowell LJ, Young RJ, Johnston ML, Tan TJ, Kleid S, Liu CS, et al. p16-positive lymph node metastases from cutaneous head and neck squamous cell carcinoma: no association with high-risk human papillomavirus or prognosis and implications for the workup of the unknown primary. Cancer. 2016;122(8):1201–1208. doi: 10.1002/cncr.29901. [DOI] [PubMed] [Google Scholar]
- 21.Yanagawa N, Wang A, Kohler D, Santos Gda C, Sykes J, Xu J, et al. Human papilloma virus genome is rare in North American non-small cell lung carcinoma patients. Lung cancer. 2013;79(3):215–220. doi: 10.1016/j.lungcan.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Kuwamoto S, Higaki H, Kanai K, Iwasaki T, Sano H, Nagata K, et al. Association of Merkel cell polyomavirus infection with morphologic differences in Merkel cell carcinoma. Hum Pathol. 2011;42(5):632–640. doi: 10.1016/j.humpath.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Leech SN, Kolar AJ, Barrett PD, Sinclair SA, Leonard N. Merkel cell carcinoma can be distinguished from metastatic small cell carcinoma using antibodies to cytokeratin 20 and thyroid transcription factor 1. J Clin Pathol. 2001;54(9):727–729. doi: 10.1136/jcp.54.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Z, Chen YY, Wu X, Trisal V, Wilczynski SP, Weiss LM, et al. Merkel cell carcinoma of lymph node with unknown primary has a significantly lower association with Merkel cell polyomavirus than its cutaneous counterpart. Mod Pathol. 2014;27(9):1182–1192. doi: 10.1038/modpathol.2013.250. [DOI] [PubMed] [Google Scholar]
- 25.Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012;67(3):395–399. doi: 10.1016/j.jaad.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Newberry TR, Kaufmann CR, Miller FR. Review of accessory parotid gland tumors: pathologic incidence and surgical management. Am J Otolaryngol. 2014;35(1):48–52. doi: 10.1016/j.amjoto.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Daniel E, McGuirt WF., Sr Neck masses secondary to heterotopic salivary gland tissue: a 25-year experience. Am J Otolaryngol. 2005;26(2):96–100. doi: 10.1016/j.amjoto.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Klubo-Gwiezdzinska J, Manes RP, Chia SH, Burman KD, Stathatos NA, Deeb ZE, et al. Clinical review: ectopic cervical thyroid carcinoma–review of the literature with illustrative case series. J Clin Endocrinol Metab. 2011;96(9):2684–2691. doi: 10.1210/jc.2011-0611. [DOI] [PubMed] [Google Scholar]
- 29.Barnes L. Surgical pathology of the head and neck. New York: CRC Press; 2008. [Google Scholar]
- 30.Doi O, Hutson JM, Myers NA, McKelvie PA. Branchial remnants: a review of 58 cases. J Pediatr Surg. 1988;23(9):789–792. doi: 10.1016/S0022-3468(88)80223-9. [DOI] [PubMed] [Google Scholar]
- 31.Golledge J, Ellis H. The aetiology of lateral cervical (branchial) cysts: past and present theories. J Laryngol Otol. 1994;108(8):653–659. doi: 10.1017/S0022215100127744. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu S, Lioe TF, Clements B. Thyroid papillary carcinoma in lateral neck cyst: missed primary tumour or ectopic thyroid carcinoma within a branchial cyst? J Laryngol Otol. 2000;114(9):716–718. doi: 10.1258/0022215001906598. [DOI] [PubMed] [Google Scholar]
- 33.Doshi J, Anari S. Branchial cyst side predilection: fact or fiction? Ann Otol Rhinol Laryngol. 2007;116(2):112–114. doi: 10.1177/000348940711600206. [DOI] [PubMed] [Google Scholar]
- 34.Guldfred LA, Philipsen BB, Siim C. Branchial cleft anomalies: accuracy of pre-operative diagnosis, clinical presentation and management. J Laryngol Otol. 2012;126(6):598–604. doi: 10.1017/S0022215112000473. [DOI] [PubMed] [Google Scholar]
- 35.Bradley PT, Bradley PJ. Branchial cleft cyst carcinoma: fact or fiction? Curr Opinion Otolaryngol Head Neck Surg. 2013;21(2):118–123. doi: 10.1097/MOO.0b013e32835cebde. [DOI] [PubMed] [Google Scholar]
- 36.Cao D, Begum S, Ali SZ, Westra WH. Expression of p16 in benign and malignant cystic squamous lesions of the neck. Hum Pathol. 2010;41(4):535–539. doi: 10.1016/j.humpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 37.LaPlante JK, Pierson NS, Hedlund GL. Common pediatric head and neck congenital/developmental anomalies. Radiol Clin North Am. 2015;53(1):181–196. doi: 10.1016/j.rcl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Soni S, Poorey VK, Chouksey S. Thyroglossal duct cyst, variation in presentation, our experience. Indian J Otolaryngol Head Neck Surg. 2014;66(4):398–400. doi: 10.1007/s12070-014-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi YM, Kim TY, Song DE, Hong SJ, Jang EK, Jeon MJ, et al. Papillary thyroid carcinoma arising from a thyroglossal duct cyst: a single institution experience. Endocr J. 2013;60(5):665–670. doi: 10.1507/endocrj.EJ12-0366. [DOI] [PubMed] [Google Scholar]
- 40.Allard RH. The thyroglossal cyst. Head Neck Surg. 1982;5(2):134–146. doi: 10.1002/hed.2890050209. [DOI] [PubMed] [Google Scholar]
- 41.Gaddikeri S, Vattoth S, Gaddikeri RS, Stuart R, Harrison K, Young D, et al. Congenital cystic neck masses: embryology and imaging appearances, with clinicopathological correlation. Curr Probl Diagn Radiol. 2014;43(2):55–67. doi: 10.1067/j.cpradiol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Deane SA, Telander RL. Surgery for thyroglossal duct and branchial cleft anomalies. Am J Surg. 1978;136(3):348–353. doi: 10.1016/0002-9610(78)90292-1. [DOI] [PubMed] [Google Scholar]
- 43.Hartl DM, Al Ghuzlan A, Chami L, Leboulleux S, Schlumberger M, Travagli JP. High rate of multifocality and occult lymph node metastases in papillary thyroid carcinoma arising in thyroglossal duct cysts. Ann Surg Oncol. 2009;16(9):2595–2601. doi: 10.1245/s10434-009-0571-9. [DOI] [PubMed] [Google Scholar]
- 44.Patel SG, Escrig M, Shaha AR, Singh B, Shah JP. Management of well-differentiated thyroid carcinoma presenting within a thyroglossal duct cyst. J Surg Oncol. 2002;79(3):134–139. doi: 10.1002/jso.10059. [DOI] [PubMed] [Google Scholar]
- 45.Ellis GL AP. Tumors of the salivary glands. Silver Spring, MD: ARP Press; 2008. [Google Scholar]
- 46.Wenig BM. Atlas of head and neck pathology. New York, NY 2008 2008.
- 47.Zhao YF, Jia Y, Chen XM, Zhang WF. Clinical review of 580 ranulas. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;98(3):281–287. doi: 10.1016/S1079-2104(04)00080-0. [DOI] [PubMed] [Google Scholar]
- 48.Pryor SG, Lewis JE, Weaver AL, Orvidas LJ. Pediatric dermoid cysts of the head and neck. Otolaryngol–Head Neck Surg. 2005;132(6):938–942. doi: 10.1016/j.otohns.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Paradis J, Koltai PJ. Pediatric teratoma and dermoid cysts. Otolaryngol Clin North Am. 2015;48(1):121–136. doi: 10.1016/j.otc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, Duran-McKinster C, Palacios-Lopez C, Ruiz-Maldonado R. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30(6):706–711. doi: 10.1111/pde.12080. [DOI] [PubMed] [Google Scholar]