Fig. 4.
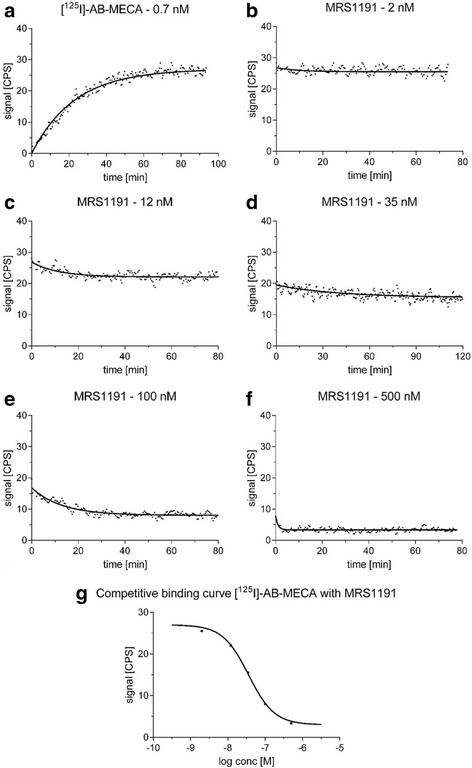
Schematic representation of the competitive real-time cell-binding experiment. Association kinetics of 0.7 nM [125I]-AB-MECA at ambient temperature to CHO-K1-hA3R (a). Association data were fitted in GraphPad Prism 6.0 using “one-phase association”. The value corresponding to the amount of bound radioligand at equilibrium was obtained using Eq. (2). Consecutive concentration-dependent dissociation kinetics of 0.7 nM [125I]-AB-MECA in the presence of 2 nM (b), 12 nM (c), 35 nM (d), 100 nM (e) and 500 nM (f) of the A3R antagonist MRS1191, respectively. Dissociation data were fitted using “dissociation—one-phase exponential decay”. Values of the remaining radioligand binding after equilibrium were obtained from Eq. (3). The competitive binding curve of [125I]-AB-MECA with MRS1191 (g) was created by means of combination of the equilibrium binding data obtained in Eqs. (2) and (3). Data were fitted using “log(inhibitor) vs. response—variable slope (four parameters)” nonlinear regression algorithm and corresponding IC50 values were obtained from Eq. (4). As different concentrations of competitor were used for each individual experiment, data in the graphs are shown from one representative experiment
