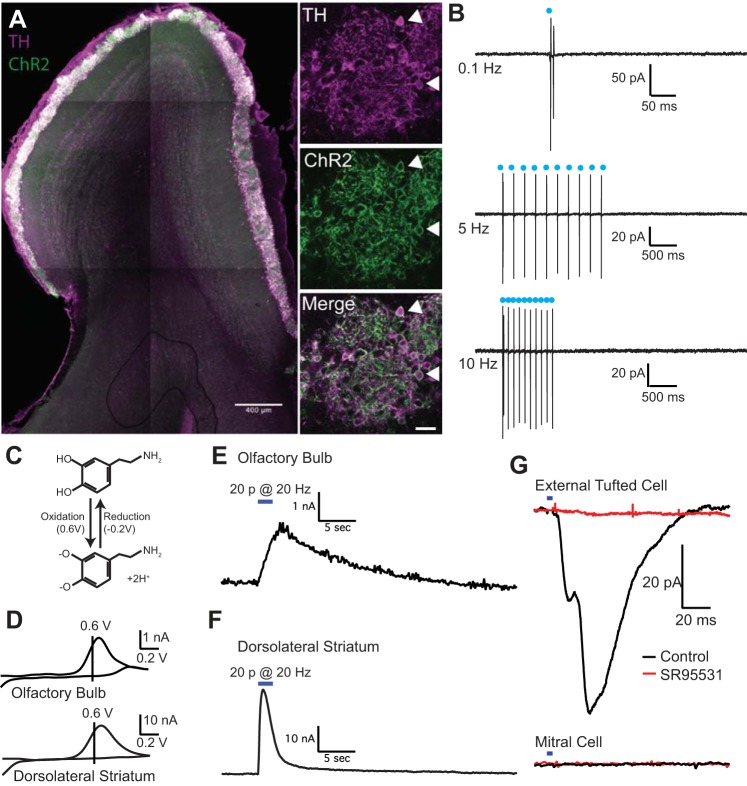
Fig. 1.
Optogenetic activation of short axon cells elicits endogenous dopamine and GABA release. A: expression of channelrhodopsin (ChR2, green) in short axon cells counterstained with tyrosine hydroxylase (TH, magenta). Double-labeled cells (white, indicated by arrowheads) were primarily located in the glomerular layer, with some TH+ external tufted cells in the juxtaglomerular external plexiform layer. B: cell-attached recordings from ChR2+ short axon cells. Optical stimulation (2 ms) reliably evoked spiking in cell-attached recordings at frequencies up to 10 Hz. C: electrochemical reaction demonstrating the cyclic oxidation and reduction of dopamine (top) to dopamine-o-quinone at characteristic voltages, which can be detected as a current with fast-scanning cyclic voltammetry. D: average cyclic voltammograms in olfactory bulb and dorsolateral striatum with oxidation and reduction peaks typical of dopamine. E and F: average oxidation current as a function of time in the olfactory bulb (E) and dorsolateral striatum (F) after 20 LED pulses at 10 Hz; the responses are plotted on the same timescale. G: optogenetic activation of short axon cells elicits a GABAA receptor mediated IPSC in external tufted cells (top, black) but not mitral cells (bottom, black). The IPSC is blocked by GABAA receptor antagonist SR95531 (red).