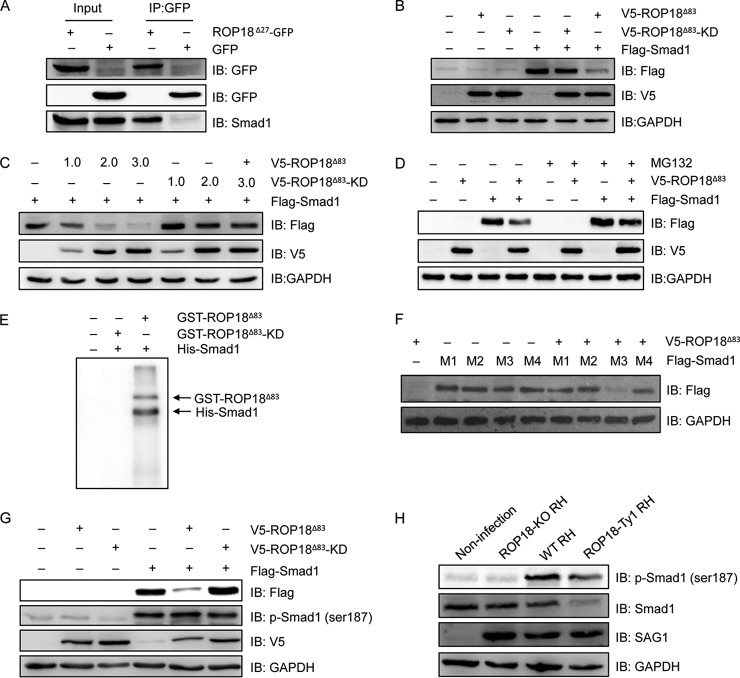
Fig. 7.
ROP18 interacts with Smad1 and triggers its proteasomal degradation via phosphorylation. A, 293T cells were transfected with ROP18Δ27-GFP expression plasmid, and coimmunoprecipitation was performed with anti-GFP, followed by immunoblotting with the indicated antibodies. IB, immunoblot. B, 293T cells were transfected with V5-ROP18Δ83 or V5-ROP18Δ83-KD and/or FLAG-Smad1 plasmids for 36 h, and then total cell lysates were analyzed by Western blotting with the indicated antibodies. C, 293T cells transiently cotransfected with the indicated amounts of V5-ROP18Δ83 or V5-ROP18Δ83-KD and FLAG-Smad1 plasmids and cell lysates were detected by Western blotting. D, 293T cells were transfected with V5-ROP18Δ83 and/or FLAG-Smad1 plasmids in the absence or presence of 10 μm MG132 for 24 h, and lysates were detected by Western blotting. E, in vitro kinase assay was performed to detected His-Smad1 phosphorylation with purified GST-ROP18Δ83 and GST-RO18Δ83-KD proteins. F, 293T cells were cotransfected with FLAG-Smad1 mutants (M1, S187A, S195A, S206A, and S214A; M2, S187A, S195A, and S206A; M3, S206A; M4, S187A) and V5-ROP18Δ83 for 48 h, and the protein levels of Smad1 mutants were detected by Western blotting. G, 293T cells were transfected with V5-ROP18Δ83 or V5-ROP18Δ83-KD and/or FLAG-Smad1 for 48 h, and Smad1 phosphorylation was detected by Western blotting with phospho-Smad1 (Ser-187) antibody. H, HFF cells were infected with wild-type (WT) RH, ROP18-KO RH, or ROP18-Ty1 RH strain, and Smad1 phosphorylation was detected by Western blotting with phospho-Smad1 (Ser-187) antibody. SAG1 and GAPDH were used as loading controls.