Figure 6. General scheme localizing the different proteoforms of LpqH into an integrated model of the lipoglycoprotein biogenesis pathway47,51.
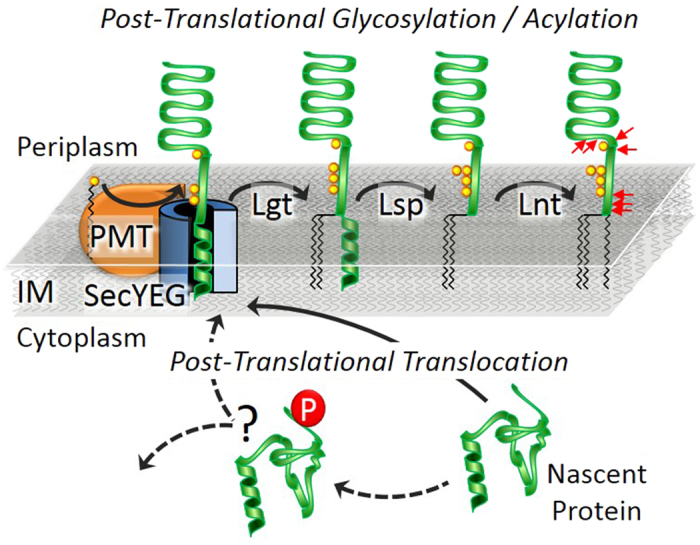
The unfolded LpqH pre-prolipoprotein, addressed via its N-terminal signal peptide (SP), to the SecYEG machinery is translocated across the inner membrane to reach the periplasmic compartment where it undergoes maturation through successive mannosylation (by the PMT and PimE) and lipidation and SP excision (by the Lgt/Lsp/Lnt enzyme triad). The absence of both glycosylation and lipidation on the phosphorylated preprolipoprotein suggests that phosphorylation occurs prior to periplasmic maturation. The early putative position of this peculiar phosphorylated proteoform in the biogenesis scheme, interrogates about its roles and its possible outcome on the lipoglycoprotein biosynthesis. (The SecA/B chaperonin protein complex has been omitted for clarity; the red arrows symbolize the N terminal extremities of the truncated forms of rLpqHHis identified herein).
