Fig. 1.
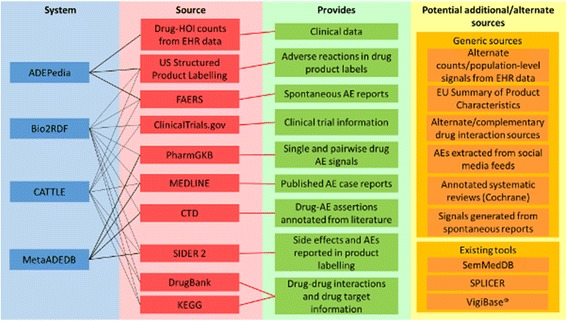
The information sources of existing knowledge-based systems for pharmacovigilance. Citations to the sources mentioned can be found in the “Background” section. EHR: electronic health record, AE: adverse event, EU: European Union, FAERS: Food and Drug Administration Adverse Event Reporting System, CTD: Comparative Toxicogenomics Database
