Fig. 13.
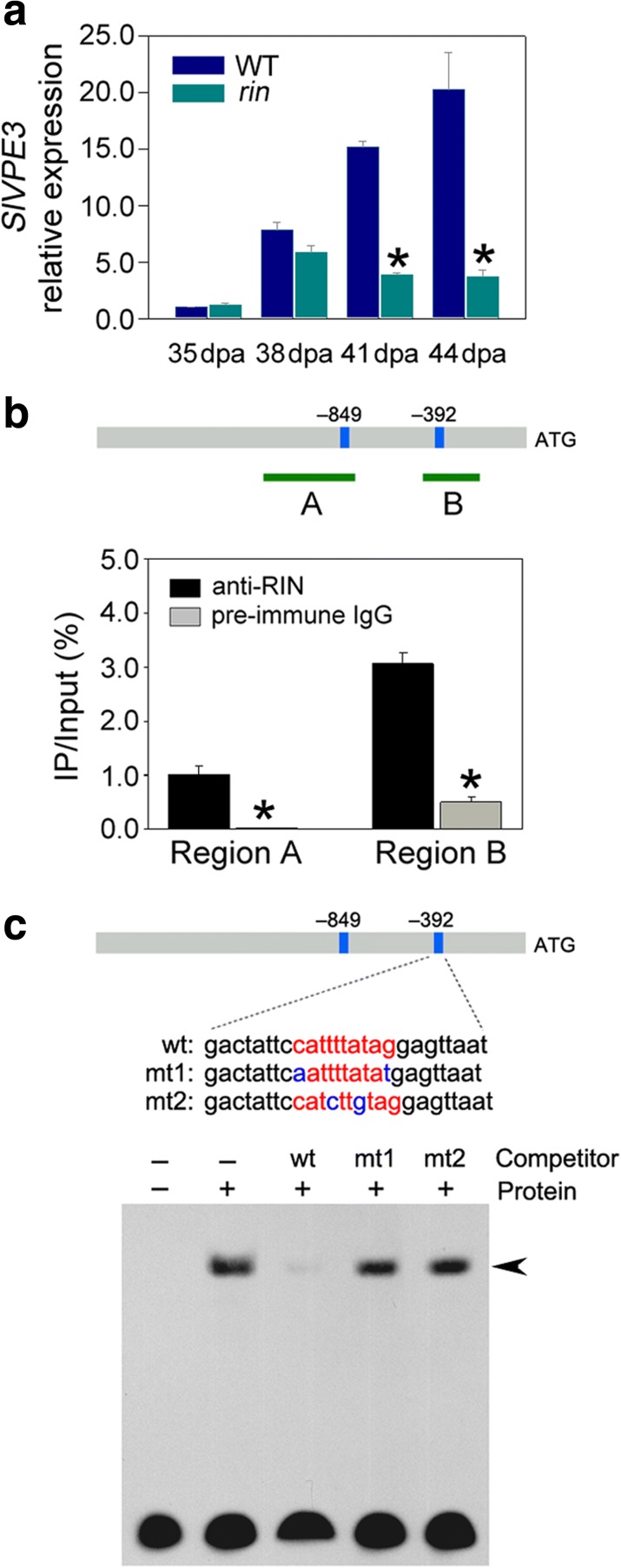
Regulation of SlVPE3 by the RIN transcription factor. a SlVPE3 expression in wild type (WT) and the rin mutant during fruit ripening, as determined by quantitative RT-PCR. The gene transcript levels were normalized against the ACTIN gene, followed by normalization against WT at 35 days post-anthesis (dpa). Values are shown as the means ± standard deviation (SD). Asterisks indicate significant differences (P < 0.05; t-test) between WT and the rin mutant. b ChIP-quantitative PCR assays indicated that RIN directly binds to the promoter of SlVPE3. The promoter structure of SlVPE3 is shown. Blue boxes represent CArG box elements and numbers indicate the position of these motifs relative to the translational start site. Green fragments with upper-case letters represent the regions used for ChIP-quantitative PCR. Values are shown as the means ± SD. Asterisks indicate significant differences (P < 0.05; t-test) between samples co-immunoprecipitated with anti-RIN antibodies and pre-immune serum. c Gel mobility shift assays revealed the direct binding of RIN to the CArG box element in the promoter region of SlVPE3. The probe sequences corresponding to the SlVPE3 promoter are shown, with red letters representing the CArG box. The mutated bases in the probes are represented by blue letters. wt, probe with intact CArG box element; mt, probe with mutated CArG box element. One thousand-fold excess amounts of unlabeled probes were added to the binding reaction as a competitor. The specific complexes formed are indicated by arrowheads