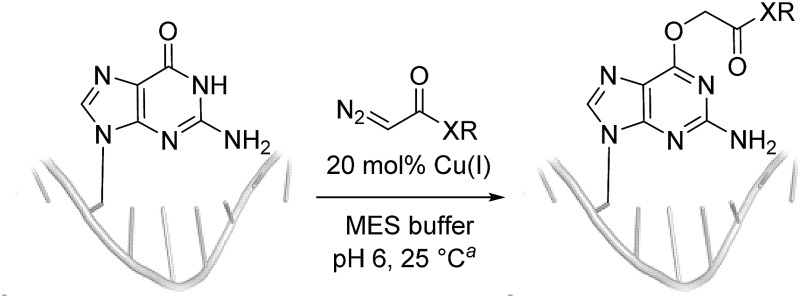
Table 2. Reactions of diazo acetamides (DAAs) with oligonucleotides.
| ||||
| Entry | Substrate | Diazo compound | Time (min) | Conv. (%) |
| 1 | d(TGT) |
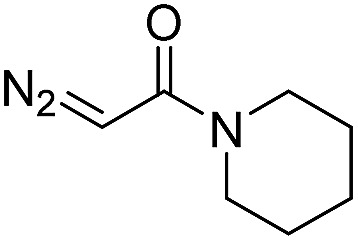
|
15 | 78 b |
| 2 | d(TGT) |
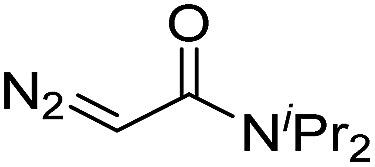
|
15 | 78 b |
| 3 | d(ATGC) |
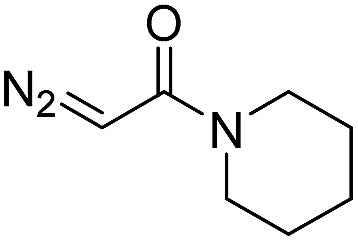
|
150 | 62 b |
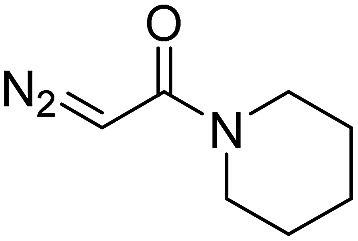
| 4 | d(ATGC) c |
|
30 | 74 b |
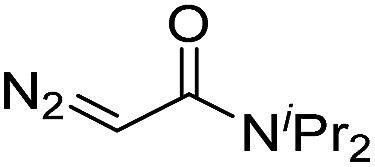
| 5 | d(ATGC) |
|
150 | 61 b |
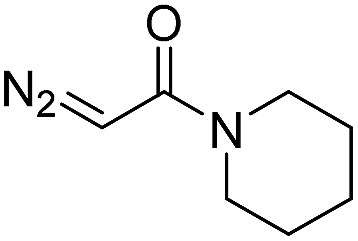
| 6 | d(TTTTGTTTT) |
|
30 | 92 b |
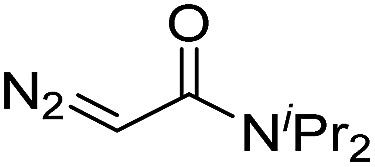
| 7 | d(TTTTGTTTT) |
|
30 | 81 b |
| 8 | d(AACAGTCATATCCTTA) |
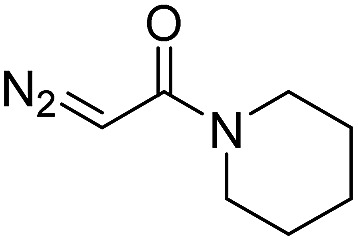
|
180 | 48 |
| 9 | d(AACAGTCATATCCTTA) |
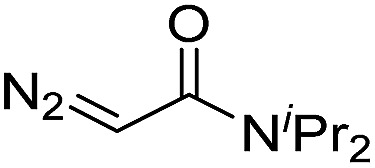
|
180 | 40 |
| 10 | d(ATGC) c |
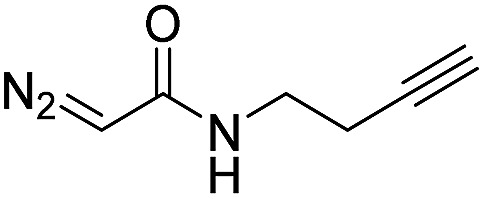
|
150 | 66 b |
aComplete conditions: 5 mM substrate, 1 mM CuSO4, 5 mM ascorbate, 50 mM diazo compound, 100 mM MES pH 6, DMSO 20% (v/v), H2O, 25 °C.
bG-alkylation was assigned on the basis of tandem MS fragmentation ions.
cSame conditions as a but with 20% (v/v) dioxane instead of DMSO.
