Figure 3.
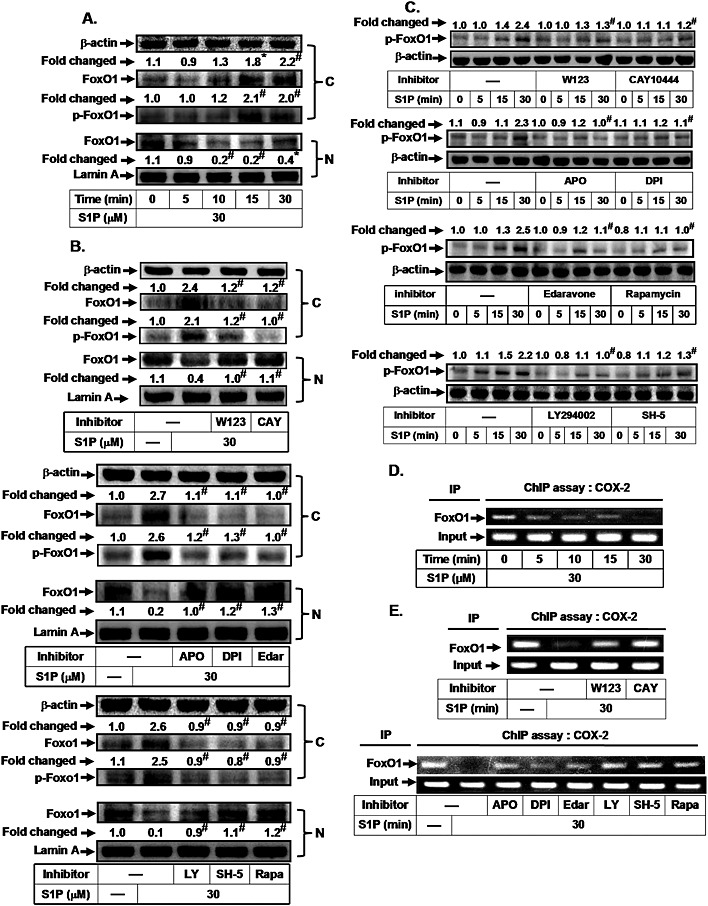
S1P induces FoxO1 activation and translocation via an mTOR‐dependent pathway. HTSMCs were (A) incubated with S1P (30 μM) for the indicated time intervals or (B) pretreated with W123, CAY10444 (CAY), apocynin (APO), DPI, edaravone (Edar), LY294002 (LY), SH‐5 or rapamycin (Rapa) for 1 h, and then incubated with S1P for 30 min. The cytosolic and nuclear fractions were prepared and subjected to Western blot analysis using an anti‐FoxO1 or anti‐phospho‐FoxO1 antibody. Lamin A and β‐actin were used as a marker protein for nuclear and cytosolic fractions, respectively. (C) Cells were pretreated without or with W123, CAY10444, apocynin, DPI, edaravone, rapamycin, LY294002 or SH‐5, and then incubated with S1P for the indicated time intervals. The levels of phospho‐FoxO1 were determined by Western blot. Cells were (D) incubated with S1P (30 μM) for the indicated time intervals or (E) pretreated with W123, CAY10444 (CAY), LY294002 (LY), SH‐5 and rapamycin (Rapa) for 1 h, or apocynin, DPI and edaravone (Edar) for 2 h, and then incubated with S1P for 30 min. The FoxO1 binding activities were analysed by a ChIP assay. Data are expressed as mean (A, B and C) of five independent experiments. *P < 0.05; # P < 0.01, as compared with the cells exposed to vehicle (A) or S1P alone (B and C).
