Abstract
Background:
The aim of this study was to determine the treatment outcome of the use of a porcine monolayer collagen matrix (mCM) to increase soft-tissue volume as a part of implant site development.
Materials and Methods:
Implants were placed in single sites in 27 patients. In the test group, mCM was used for soft-tissue augmentation. No graft was placed in the control group. Soft-tissue thickness (STTh) was measured at the time of surgery (T0) and 6 months postoperatively (T1) at two sites (STTh 1, 1 mm below the gingival margin; STTh 2, 3 mm below the mucogingival margin).
Results:
Significant increases (P < 0.001) in STTh (STTh 1 = 1.06 mm, 117%; STTh 2 = 0.89 mm, 81%) were observed in the test group. Biopsy results showed angiogenesis and mature connective tissue covered by keratinized epithelium.
Conclusions:
Within the limitations of this study, it could be concluded that mCM leads to a significant increase of peri-implant soft-tissue thickness, with good histological integration and replacement by soft tissue and may serve as an alternative to connective tissue grafting.
Key words: Augmentation, collagen matrix, connective tissue, tissue thickness, tissue volume
INTRODUCTION
Different approaches to the preservation or augmentation of soft and hard oral tissue amounts have been described. Implant placement immediately after extraction is used to maximize the preservation of these tissues. However, certain amounts of buccal bone wall resorption and soft-tissue recession may occur, leading to an unfavorable esthetic result.[1,2,3]
Soft tissue augmentation can be successfully performed with the use of autogenous connective tissue. The use of connective tissue grafts (CTGs) prior, during, or after implant placement can lead to an increase in soft-tissue thickness (STTh).[4,5,6] The main disadvantages of CTGs are the need for a second surgical site, increasing morbidity and postoperative discomfort, and the limited amount of tissue that can be harvested. Collagen-based membranes have been used in periodontal and implant regenerative surgery as barriers to prevent epithelial migration and allow cells with regenerative capacity to repopulate the defect area.[7] Recently, collagen-based matrices (CMs) have been introduced for soft-tissue augmentation.[8] Although these materials have been used to increase the width of keratinized soft-tissue, no clinical study has examined the use of CMs to enhance peri-implant STTh in humans.[6,9,10,11] Thus, the aim of this study was to determine the outcome of treatment using a new monolayer CM (mCM) instead of autogenous connective tissue to increase soft-tissue volume as a part of implant site development.
MATERIALS AND METHODS
Patients
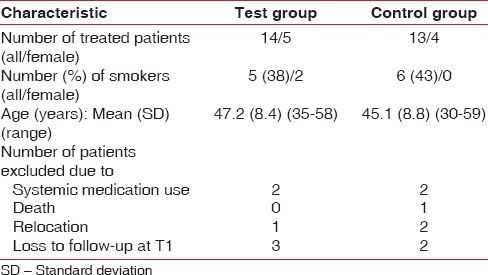
A total of 27 sites in 27 patients (18 males, 9 females; mean age 46.11 years, range 30–59 years) treated from September 2011 to April 2013 were included in this prospective, randomized, examiner-blinded, controlled clinical study [Table 1]. Patients were treated in the Division of Periodontology, Catholic University, Rome, Italy. Only patients requiring single implants in the premolar area of the maxilla were included. Exclusion criteria were implant treatment needs in the mandible, pregnancy, diabetes mellitus, systemic medication use, drug abuse, and smoking ≥10 cigarettes/day.
Table 1.
Demographic characteristics of the study population
The study was approved by the Institution's Ethics Commission (P/438/CF/2009) and performed in accordance with the Helsinki Declaration of 1975, as revised in 2000. Recruited patients were given a full description of the study, including the surgical procedures, and then given at least 2 weeks to submit a signed informed consent form.
Randomization and treatment
The first examiner performed tooth extractions and periodontal treatment at least 6 months before the study began. Using randomization software (Studie Berechnung; Unisolo, Braunschweig, Germany) a second examiner assigned patients to the test group, in which mCMs were used for soft-tissue augmentation, and the control group, which received no augmentative procedure.
A third examiner performed all surgeries. Target areas for implant placement were edentulous, with no history of socket preservation [Figure 1]. Cylindrical screw-type implants (Dentegris, Duisburg, Germany) (diameter 3.75 mm, length 10 or 11.5 mm) were placed manually (875 rpm, 35 Ncm torque) using a two-stage surgical approach.
Figure 1.
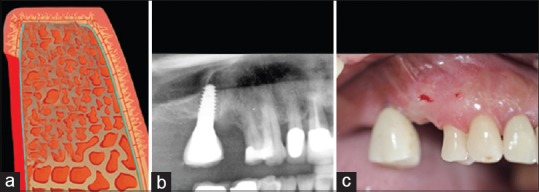
The target areas were healed without socket preservation; (a) Schematic drawing. (b) X-ray; (c) Clinical view
In the test group, implant placement was performed following the performance of a midcrestal incision and vertical releasing incisions on the face and elevation of a split-thickness flap [Figure 2]. A porcine mCM (Mucoderm® [CE 0483]; Botiss Biomaterials, Berlin, Germany) of 1.7 mm thickness (approved in Europe for soft-tissue augmentation) was used for augmentation [Figure 3]. For each patient, an mCM was hydrated in sterile saline solution for 10 min, trimmed, and positioned on the periosteum to extend from the free gingival margin to 5 mm below the mucogingival junction. It was then fixated with interrupted simple-loop resorbable sutures (5-0 Monocryl; Johnson and Johnson International, Neuss, Germany). The mucosal flap was repositioned to cover the matrix and fixed using nonresorbable sutures (4-0 Ethibond Excel; Johnson and Johnson International) [Figure 4].
Figure 2.

Surgery in the test group. (a) Schematic drawing of flap elevation; (b) Clinical view
Figure 3.
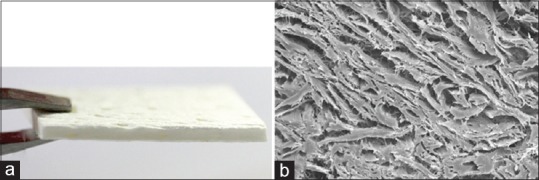
(a) The monolayer collagen matrix used in this study; (b) Electron microscopic image showing the regularly organised collagen network
Figure 4.
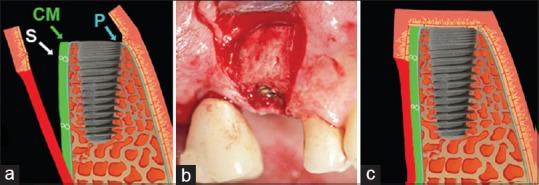
Grafting using the monolayer collagen matrix (test group). (a) Schematic drawing of monolayer collagen matrix placement on the periosteum (P) with resorbable sutures (S); (b) Clinical view at the time of monolayer collagen matrix placement; (c) Flap repositioning
In the control group, a midcrestal incision was made at the implant site, and a full-thickness flap extending only over the area of implant bed preparation was elevated without the creation of releasing incisions. The implant bed was then prepared [Figure 5]. Following implant placement, the mucosal flap was repositioned and sutured [Figure 5c].
Figure 5.

Schematic drawings of surgery in the control group. (a) Minimally invasive elevation of the mucosa over the implant site only, without flap elevation; (b) Implant placement; (c) Flap repositioning
Measurements
STTh measurements were performed preoperatively (T0) and 6 months postoperatively (T1), before implants were uncovered and healing abutments were positioned. To standardize measurement, a customized metal stent (CrCo alloy) was fabricated with a metal plate overlying the surgical area with a distance of 8 mm. The plate had two holes; one hole was positioned 1 mm below the gingival margin (for STTh 1 measurement), and the other was positioned 3 mm below the mucogingival margin (for STTh 2 measurement) [Figure 6]. Using light pressure, endodontic reamers (#15; Dentsply DeTrey, Konstanz, Germany) with silicone disc stops were inserted through these holes and perpendicularly through the mucosal surface to the cortical bone. The stops were then placed in tight contact with the soft-tissue surface and fixated with cyanoacrylate [Figure 7]. After careful removal of the stent with the reamer in place, penetration depth was measured at both sites to the nearest 0.1 mm using a gauge. Measurements at T1 were performed using the customized stents before implant uncovering [Figure 8].
Figure 6.
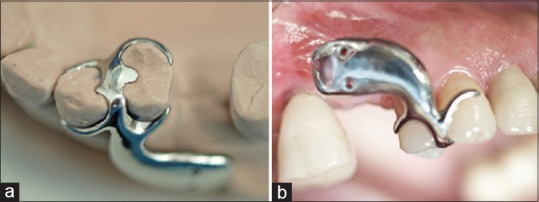
Use of the measurement stent. (a) Occlusal view on the master cast; (b) Intraoral lateral view of measurement taking
Figure 7.

Measurements. (a) Schematic drawings of soft-tissue thickness measurement at T0, R: Endodontic reamers, ST: Stent, SI: Silicon stops; (b) Clinical view of soft-tissue thickness measurements
Figure 8.
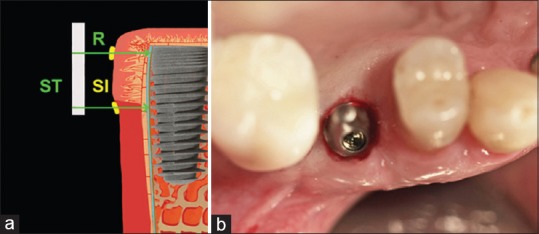
Soft-tissue thickness measurement at T1 and implant uncovering. (a) Schematic drawing: Endodontic reamers (R), inserted through soft-tissue until bone contact, guided by the stent (ST) with silicon stops (SI); (b) Clinical view of soft-tissue condition after the implant was uncovered
Histology
Soft tissue biopsy samples were collected at T1 after STTh measurement, and submitted for histological evaluation. Half of each tissue sample was stained with hematoxylin and eosin, and the other half was stained with alpha-smooth-muscle actin (αSMA). Samples were examined under a light microscope.
Postoperative care and restorative procedure
Patients were prescribed a chlorhexidine mouth rinse (0.1% chlorhexamed fluid; GlaxoSmithKline, Buehl, Germany) twice daily for 2 weeks. Sutures were removed at 2 weeks. The implants were uncovered and loaded 6 months after placement (T1). Porcelain fused metal crowns were fabricated and fixed using temporary cement (TempBond; Kavo Kerr Group, Charlotte, NC, USA).
Data analysis
Anonymized data were sent to a statistical institute not associated with the university for analysis (Statsconsultancy Ltd., Amersham, UK) Demographic characteristics and baseline measurements of the two groups were compared. Continuous variables were found to be distributed normally and were compared using unpaired t-tests. Fisher's exact test was used to compare categorical variables.
Absolute (in millimeters) and percentage changes in STTh from T0 to T1 were evaluated. Within-group absolute and relative changes were analyzed using paired t-tests and one-sample t-tests, respectively. Between-group differences in both measures were examined using unpaired t-tests. Paired t-tests were used to compare changes at STTh measurement sites 1 and 2. Pearson correlation was used to examine associations of the treatment outcome with age, and unpaired t-tests were used to examine associations with sex and graft position.
RESULTS
Eleven of 38 recruited patients (6 in the test group, 7 in the control group) were excluded during the study period. Analyses thus included 27 sites in 27 patients (18 men, 9 women; mean age 46.11 [range 30–59] years) [Table 1]. No significant difference in sex, implant position (left/right maxilla), baseline age, or baseline smoking status was observed between groups. At T0, STTh was lesser in the test group than in the control group (1.13 mm vs. 1.65 mm, P < 0.001) [Table 2]. No complication or implant failure was observed during the study period. Patients reported minimal pain and swelling in the grafted area.
Table 2.
Within- and between-group comparisons of changes in soft-tissue thickness from T0 (time of surgery) to T1 (6 months postoperatively)
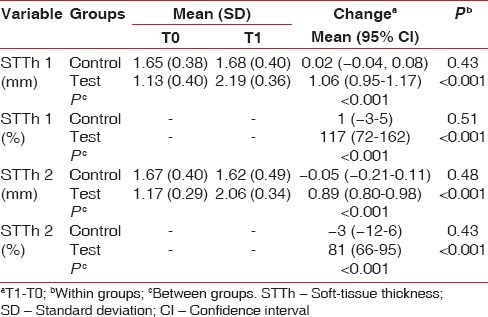
STTh 1 and STTh 2 increased significantly from T0 to T1 in the test group, by 117% (1.1 mm) and 81% (0.9 mm), respectively [Table 2]. No such change was observed in the control group. Changes from T0 to T1 thus differed significantly between the test and control groups [Table 2]. No significant difference according to demographic characteristics or graft position was detected.
Biopsy samples showed multi-layered epithelium with physiologically shaped rete pegs at all sites [Figure 9]. In the submucosal layer, connective tissue rich in collagen fibers with only localized areas of inflammatory cells was present. No mCM remnant was detected; the material had been resorbed completely and replaced by host connective tissue. Multitudes of capillaries and larger blood vessels were observed. The walls of these vessels showed αSMA-antibody positivity and were thus classified as arterioles.
Figure 9.
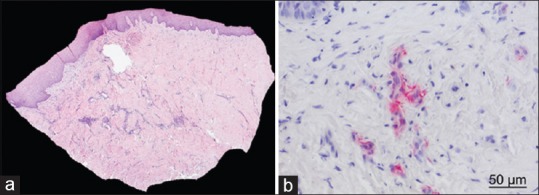
Histological sections. (a) Connective tissue and well-keratinised epithelium are visible (H and E, ×25); (b) Capillaries and larger blood vessels are visible. Vessel walls reacted positively to alpha-smooth-muscle actin (Alpha smooth muscle actin staining, ×400)
DISCUSSION
In this study, mCMs were used as a grafting material alternative to host connective tissue for peri-implant soft tissue augmentation. The procedure resulted in minimal pain and swelling, and a mean increase in STTh of 1.06 mm was obtained. This increase is only slightly less than reported with the use of CTGs and different surgical techniques (1.75 mm and 1.3 mm).[12,13]
Although STTh does not compromise the mechanical stability of implants, it is correlated negatively with peri-implant soft tissue recession.[14,15,16] To prevent negative esthetic outcomes, STTh-enhancing peri-implant soft tissue augmentation techniques have been developed; the majority of them are performed at the time of implant placement.[5,17,18,19,20,21,22] To obtain a significant increase in tissue volume, large grafts and/or the use of tissue folding procedures is necessary.[4,7]
According to the manufacturer, the mCM used in the present study was made without cross-linking and was processed to remove antigenic cellular components without damaging the tissue structure, thereby preserving the structural integrity of the entire extracellular CM. In an animal model, the rough and porous collagen structure was shown to serve as a scaffold for ingrowing blood vessels and cells, enabling revascularization of the matrix and its integration into the surrounding tissue with progressive complete remodeling.[9,10,11] This property may explain the good integration, demonstrated by histological findings, observed in this study. Biopsy samples from all sites showed evidence of angiogenesis and mature connective tissue covered by keratinized epithelium, with clinical gains in mucosal thickness ranging from 0.7 to 1.5 mm.
In vitro response of four different lines of oral cells to the mCM used in this study demonstrated good biocompatibility. Subcutaneous implantation of the mCM into mice resulted in good integration, with tissue ingrowth and significant revascularization of its collagen structure.[23] Close contact between the mCM and underlying periosteum, and good fixation to avoid micro-movement that might destroy the newly formed blood vessel network, thus appear to be advisable.
Within the limitations of this study, we conclude that mCM use leads to a significant increase in soft-tissue volume, with good histological integration and replacement of the graft material by soft-tissue. This procedure obviates the need to harvest autogenous CTGs s from the palate. Further controlled clinical studies with larger samples are needed to validate these findings and to establish the best treatment protocol.
Within the limitations of this study, it could be concluded that mCM leads to a significant increase of peri-implant soft-tissue thickness, with good histological integration and replacement by soft tissue and may serve as an alternative to connective tissue grafting.
Financial support and sponsorship
Collagen matrices were provided by Botiss Biomaterials. Dental implants were provided by Dentegris Germany.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank the companies Botiss Biomaterials and Dentegris for supporting this study by providing their products.
REFERENCES
- 1.Cornelini R, Barone A, Covani U. Connective tissue grafts in postextraction implants with immediate restoration: A prospective controlled clinical study. Pract Proced Aesthet Dent. 2008;20:337–43. [PubMed] [Google Scholar]
- 2.de Sanctis M, Vignoletti F, Discepoli N, Zucchelli G, Sanz M. Immediate implants at fresh extraction sockets: Bone healing in four different implant systems. J Clin Periodontol. 2009;36:705–11. doi: 10.1111/j.1600-051X.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 3.Botticelli D, Renzi A, Lindhe J, Berglundh T. Implants in fresh extraction sockets: A prospective 5-year follow-up clinical study. Clin Oral Implants Res. 2008;19:1226–32. doi: 10.1111/j.1600-0501.2008.01620.x. [DOI] [PubMed] [Google Scholar]
- 4.Zuhr O, Bäumer D, Hürzeler M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J Clin Periodontol. 2014;41(Suppl 15):S123–42. doi: 10.1111/jcpe.12185. [DOI] [PubMed] [Google Scholar]
- 5.Kan JY, Rungcharassaeng K, Lozada JL. Bilaminar subepithelial connective tissue grafts for immediate implant placement and provisionalization in the esthetic zone. J Calif Dent Assoc. 2005;33:865–71. [PubMed] [Google Scholar]
- 6.Thoma DS, Hämmerle CH, Cochran DL, Jones AA, Görlach C, Uebersax L, et al. Soft tissue volume augmentation by the use of collagen-based matrices in the dog mandible – A histological analysis. J Clin Periodontol. 2011;38:1063–70. doi: 10.1111/j.1600-051X.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 7.Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE. Collagen as an implantable material in medicine and dentistry. J Oral Implantol. 2002;28:220–5. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Thoma DS, Benic GI, Zwahlen M, Hämmerle CH, Jung RE. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009;20(Suppl 4):146–65. doi: 10.1111/j.1600-0501.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 9.Rothamel D, Benner M, Fienitz T, Happe A, Kreppel M, Nickenig HJ, et al. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices – An experimental study in the rat. Head Face Med. 2014;10:10. doi: 10.1186/1746-160X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanaati S, Schlee M, Webber MJ, Willershausen I, Barbeck M, Balic E, et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 2011;6:015010. doi: 10.1088/1748-6041/6/1/015010. [DOI] [PubMed] [Google Scholar]
- 11.Rocchietta I, Schupbach P, Ghezzi C, Maschera E, Simion M. Soft tissue integration of a porcine collagen membrane: An experimental study in pigs. Int J Periodontics Restorative Dent. 2012;32:e34–40. [PubMed] [Google Scholar]
- 12.Speroni S, Cicciu M, Maridati P, Grossi GB, Maiorana C. Clinical investigation of mucosal thickness stability after soft tissue grafting around implants: A 3-year retrospective study. Indian J Dent Res. 2010;21:474–9. doi: 10.4103/0970-9290.74208. [DOI] [PubMed] [Google Scholar]
- 13.Wiesner G, Esposito M, Worthington H, Schlee M. Connective tissue grafts for thickening peri-implant tissues at implant placement. One-year results from an explanatory split-mouth randomised controlled clinical trial. Eur J Oral Implantol. 2010;3:27–35. [PubMed] [Google Scholar]
- 14.Zigdon H, Machtei EE. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin Oral Implants Res. 2008;19:387–92. doi: 10.1111/j.1600-0501.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy JE, Bird WC, Palcanis KG, Dorfman HS. A longitudinal evaluation of varying widths of attached gingiva. J Clin Periodontol. 1985;12:667–75. doi: 10.1111/j.1600-051x.1985.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 16.Wennström JL. Lack of association between width of attached gingiva and development of soft tissue recession. A 5-year longitudinal study. J Clin Periodontol. 1987;14:181–4. doi: 10.1111/j.1600-051x.1987.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 17.Kan JY, Rungcharassaeng K, Lozada JL, Zimmerman G. Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: A 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 2011;26:179–87. [PubMed] [Google Scholar]
- 18.Kan JY, Rungcharassaeng K, Morimoto T, Lozada J. Facial gingival tissue stability after connective tissue graft with single immediate tooth replacement in the esthetic zone: Consecutive case report. J Oral Maxillofac Surg. 2009;67(11 Suppl):40–8. doi: 10.1016/j.joms.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Yoshino S, Kan JY, Rungcharassaeng K, Roe P, Lozada JL. Effects of connective tissue grafting on the facial gingival level following single immediate implant placement and provisionalization in the esthetic zone: A 1-year randomized controlled prospective study. Int J Oral Maxillofac Implants. 2014;29:432–40. doi: 10.11607/jomi.3379. [DOI] [PubMed] [Google Scholar]
- 20.Rungcharassaeng K, Kan JY, Yoshino S, Morimoto T, Zimmerman G. Immediate implant placement and provisionalization with and without a connective tissue graft: An analysis of facial gingival tissue thickness. Int J Periodontics Restorative Dent. 2012;32:657–63. [PubMed] [Google Scholar]
- 21.Tsuda H, Rungcharassaeng K, Kan JY, Roe P, Lozada JL, Zimmerman G. Peri-implant tissue response following connective tissue and bone grafting in conjunction with immediate single-tooth replacement in the esthetic zone: A case series. Int J Oral Maxillofac Implants. 2011;26:427–36. [PubMed] [Google Scholar]
- 22.Chung S, Rungcharassaeng K, Kan JY, Roe P, Lozada JL. Immediate single tooth replacement with subepithelial connective tissue graft using platform switching implants: A case series. J Oral Implantol. 2011;37:559–69. doi: 10.1563/AAID-JOI-D-10-00110. [DOI] [PubMed] [Google Scholar]
- 23.Pabst AM, Happe A, Callaway A, Ziebart T, Stratul SI, Ackermann M, et al. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J Periodontal Res. 2014;49:371–81. doi: 10.1111/jre.12115. [DOI] [PubMed] [Google Scholar]


