Figure 1. The deubiquitinase Usp27x interacts with BimEL .
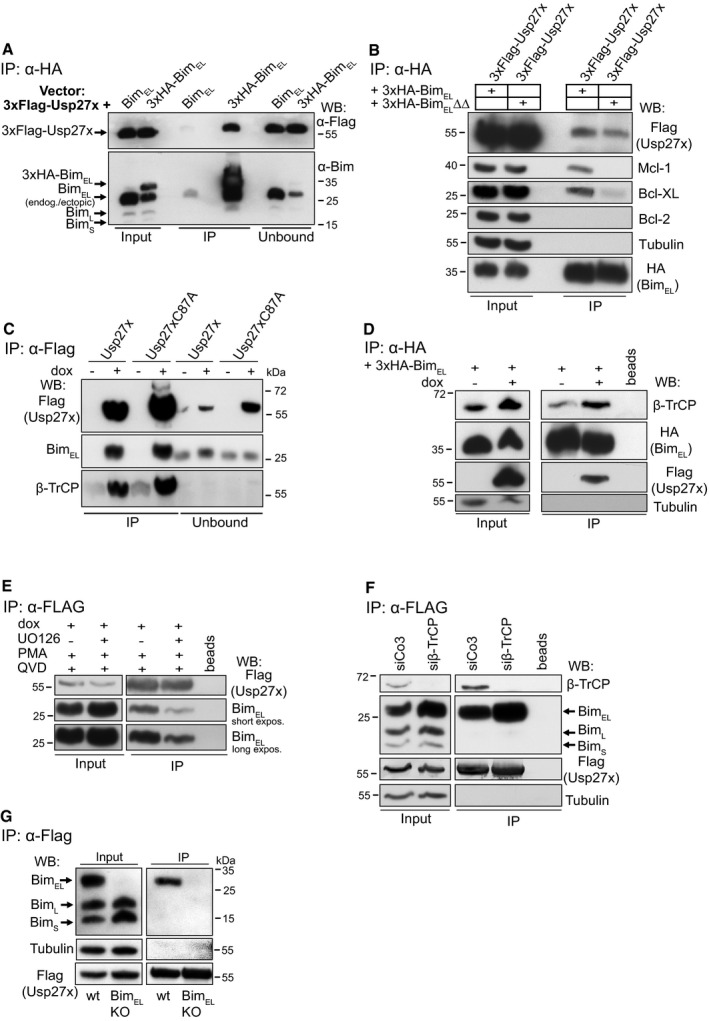
- 293FT cells were transfected with 3xFlag‐Usp27x (pFCMV7.1 vector backbone) together with a construct driving expression of untagged BimEL or 3xHA‐BimEL (both pMIG‐vector backbone). Cells were lysed, and 3xHA‐BimEL was immunoprecipitated with anti‐HA antibodies. Immunoprecipitation products were tested by Western blotting for the presence of Bim and FLAG‐Usp27x probing with antibodies against Bim or against the FLAG‐peptide. See also Fig EV1B. Western blots show representative of n ≥ 3 independent experiments.
- Usp27x binds a mutant of Bim incapable of binding to anti‐apoptotic Bcl‐2 proteins. 293FT cells transfected with constructs encoding 3xFLAG‐Usp27x and 3xHA‐tagged BimEL (see A, 2 μg each) or 3xHA‐tagged BimEL∆∆ (a mutant with two mutations in the BH3 domain, incapable of binding anti‐apoptotic Bcl‐2 proteins 50) were immunoprecipitated from whole‐cell extracts using anti‐HA resin. Bim and Usp27x were detected with anti‐HA and anti‐FLAG antibodies as indicated; anti‐apoptotic proteins: Mcl‐1, Bcl‐XL, Bcl‐2. The caspase inhibitor Q‐VD‐OPh (QVD) was added to the cultures described in (A) and (B) to inhibit Bim‐induced apoptosis. Western blots are representative of n = 3 independent experiments.
- Usp27x interacts with endogenous BimEL independently of its catalytic activity. 293FT cells either carrying 3xFlag‐Usp27x (293FT‐TetR‐3xFlag‐Usp27x) or the catalytically inactive mutant 3xFlag‐Usp27xC87A under the control of the Tet repressor (TetR) were treated for 24 h with doxycycline (dox) to induce expression of Usp27x or Usp27xC87A. In all conditions, PMA (to induce Bim ubiquitination, 16.2 nM) and Q‐VD‐OPh (to inhibit apoptosis, 10 μM, see Fig 3) were added at the time of Usp27x induction. MG132 (to prevent Bim degradation, 40 μM) was added in all conditions 4 h prior to cell lysis. 3xFlag‐tagged Usp27x or Usp27xC87A was immunoprecipitated from whole‐cell lysates using anti‐Flag resin. Interaction with BimEL or β‐TrCP was detected by Western blotting using anti‐Bim or anti‐β‐TrCP antibodies. Western blots are representative of n ≥ 3 independent experiments.
- Usp27x expression does not inhibit interaction of BimEL to β‐TrCP. 293FT‐TetR‐3xFlag‐Usp27x cells were transfected with pMIG‐3xHA‐BIMEL in the presence of both PMA and QVD. At the same time, dox (to induce 3xFlag‐Usp27x) was added as indicated and 20 h later cells were treated with MG132 (40 μM) for additional 4 h. Cells were lysed and 3xHA‐BimEL was immunoprecipitated as described above. As a control, HA matrix was used without lysate to rule out any unspecific signal that might come from the immobilized anti‐HA antibody (beads). Western blots show representative of n = 2 independent experiments.
- Binding of Usp27x to BimEL can be blocked by the MEK inhibitor UO126. 293FT‐TetR‐3xFlag‐Usp27x cells (see C) were treated for 24 h with dox to induce expression of Usp27x. In all conditions, PMA (16.2 nM) and Q‐VD‐OPh (10 μM) were added at the time of Usp27x induction. As a control, cells were pre‐treated with UO126 (10 μM) for 30 min to block the PMA‐stimulated ERK pathway before the addition of dox plus PMA plus QVD. 3xFlag‐tagged Usp27x was immunoprecipitated from whole‐cell lysates using anti‐Flag resin. Interaction with BimEL was detected by Western blotting using anti‐Bim antibodies. As a control, Flag‐matrix (beads) was used alongside the IP under same conditions but without addition of protein lysates. Western blots are representative of n = 4 independent experiments (see also Fig EV2B).
- Binding of Usp27x to BimEL does not require β‐TrCP. 293FT‐TetR‐3xFlag‐Usp27x cells were transfected with control siRNA (siCo3) or siRNA specific for β‐TrCP. Forty‐eight hours later, cells were stimulated with PMA plus dox plus QVD for additional 24 h. After cell lysis, Flag‐Usp27x was immunoprecipitated using anti‐Flag‐matrix, and BimEL and β‐TrCP bound to Usp27x were identified by Western blotting. Blots are representative of n = 2 independent experiments.
- Usp27x binds specifically to BimEL. 293FT‐TetR‐3xFlag‐Usp27x cells or 293FT‐3xFlag‐Usp27x cells with a specific deletion of the BimEL protein were treated with dox, PMA and QVD as in (C). Cells were lysed, and lysates were subjected to anti‐Flag immunoprecipitation. 3xFlag‐Usp27x was detected using anti‐FLAG antibodies. Blots are representative of n = 3 independent experiments.
