Figure 2. Extensive PALB2 phosphorylation after DNA damage requires three N‐terminal ATM/ATR consensus target sites.
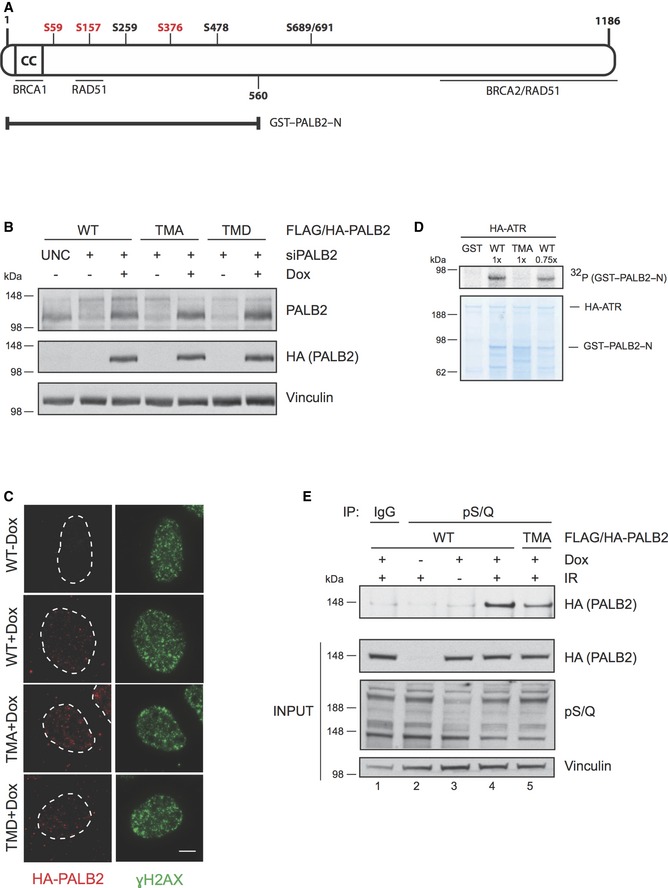
- Schematic figure of the human PALB2 protein. The N‐terminus of PALB2 contains a coiled‐coil region (CC) that mediates the interaction between PALB2 and BRCA1. PALB2 interacts with BRCA2 via the C‐terminal WD40 domain, which also harbors interaction sites for RAD51. An additional region for RAD51 interaction is located in the N‐terminus at amino acid residues 101–184. PALB2 has seven potential S/Q phosphorylation sites of which serines S59, S157, and S376 (depicted in red) were mutated either to alanine (triple mutant S to A, TMA) to create a phospho‐deficient mutant, or aspartic acid (triple mutant S to D, TMD) for a phospho‐mimicking mutant. GST–PALB2–N (amino acids 1‐560) contains the N‐terminal serines.
- Characterization of the doxycycline‐inducible cell lines expressing siRNA‐resistant wild‐type (WT) or mutant (TMA or TMD) FLAG/HA‐tagged PALB2. The cell lines were transfected with UNC (negative control) or PALB2 siRNA for 24 h prior to induction with doxycycline. Immunoblots were performed with the indicated antibodies.
- Cell line characterization of FLAG/HA‐tagged PALB2 localization 2 h following exposure to 15 Gy of IR. Z‐stack max intensity image projection and background subtraction was done using ImageJ/Fiji. Scale bar = 5 μm.
- Kinase assay was performed as described in Fig 1E except that the SDS–PAGE gel was Coomassie stained following electrophoresis. WT was used at two different concentrations (1× and 0.75×).
- Doxycycline‐inducible stable U2OS cell lines were left untreated or treated with doxycycline and/or IR (15 Gy, 2 h recovery). Following protein extraction, the lysates were immunoprecipitated (IP) with IgG or antibody recognizing phosphorylated S/Q sites (pS/Q) and analyzed by SDS–PAGE. Immunoblots were performed with the indicated antibodies.
