Abstract
The human oropharyngeal muscles have a unique anatomy with diverse and intricate functions. To investigate if this specialization is also reflected in the cytoarchitecture of muscle fibers, intermediate filament proteins and the dystrophin‐associated protein complex have been analyzed in two human palate muscles, musculus uvula (UV) and musculus palatopharyngeus (PP), with immunohistochenmical and morphological techniques. Human limb muscles were used as reference. The findings show that the soft palate muscle fibers have a cytoskeletal architecture that differs from the limb muscles. While all limb muscles showed immunoreaction for a panel of antibodies directed against different domains of cytoskeletal proteins desmin and dystrophin, a subpopulation of palate muscle fibers lacked or had a faint immunoreaction for desmin (UV 11.7% and PP 9.8%) and the C‐terminal of the dystrophin molecule (UV 4.2% and PP 6.4%). The vast majority of these fibers expressed slow contractile protein myosin heavy chain I. Furthermore, an unusual staining pattern was also observed in these fibers for β‐dystroglycan, caveolin‐3 and neuronal nitric oxide synthase nNOS, which are all membrane‐linking proteins associated with the dystrophin C‐terminus. While the immunoreaction for nNOS was generally weak or absent, β‐dystroglycan and caveolin‐3 showed a stronger immunostaining. The absence or a low expression of cytoskeletal proteins otherwise considered ubiquitous and important for integration and contraction of muscle cells indicate a unique cytoarchitecture designed to meet the intricate demands of the upper airway muscles. It can be concluded that a subgroup of muscle fibers in the human soft palate appears to have special biomechanical properties, and their unique cytoarchitecture must be taken into account while assessing function and pathology in oropharyngeal muscles.
Keywords: cytoskeleton, desmin, dystrophin, muscle fiber, palatopharyngeus, sleep apnea, soft palate, uvula
Introduction
The human soft palate is involved in diverse and intricate oropharyngeal functions, such as respiration, speech, swallowing and ventilation of the ear. Five pairs of muscles contribute to the formation and movements of the soft palate. Three of these, musculus uvula (UV), tensor veli palatini and levator veli palatini arise from bony attachment in the skull, while two, musculus palatopharyngeus (PP) and palatoglossus, ascend from the pharyngeal walls and the tongue, respectively. This special anatomy with lack of firm attachment at least at one end is a trait that palate muscles share with orofacial muscles. It has previously been shown that each of the palate muscles has a distinct morphological and molecular identity, and that they generally have more structural similarities with orofacial than with the limb muscles (Stål et al. 1987, 1990, 1994, 1995; Kuehn & Kahane, 1990; Stål & Lindman, 2000). In recent years, the muscles of the soft palate have generated interest among researchers studying their role in sleep‐disordered breathing as well as in the pathophysiology of swallowing and speech disorders.
The internal framework of the muscle cell, the cytoskeleton, is composed of a network of protein filaments that extends throughout the cytosol of the cell. These proteins play an important role in sarcomeric movements by linking the contractile apparatus to the sarcolemma and extracellular matrix (ECM). The cytoskeleton also has important roles in cell shape, signal transduction, growth, division and differentiation, as well as in the movement of organelles within the cell (Capetanaki et al. 2007). In skeletal muscles the major elements of the cytoskeleton are the intermediate filaments (IFs; Paulin & Li, 2004). Desmin is the most abundant IF in mature muscles, and it plays a central role in the integration of structure and function of the muscles. This protein is located at the periphery of the Z‐disc and links the entire contractile apparatus to the subsarcolemmal cytoskeleton, the cell nuclei and to other organelles (Small et al. 1992; Fuchs & Weber, 1994). The network of desmin filaments helps in maintaining the structural and mechanical integrity of the cell during contraction, contributes to force transmission and longitudinal load bearing (Paulin & Li, 2004; Shah et al. 2004).
Another vital protein that connects the cytoskeleton of a muscle fiber to the surrounding ECM through the cell membrane is dystrophin (Campbell, 1995; Srivastava & Yu, 2006). Dystrophin binds to the membrane‐spanning dystrophin‐associated protein complex (DAPC) through its C‐terminus domain, whereas its N‐terminus interacts with actin filaments. The DAPC is composed of membrane‐related proteins, such as dystroglycans, sarcoglycans, caveolin‐3, neuronal nitric oxide synthase (nNOS) and laminin containing the α‐2 chain (Davies & Nowak, 2006). The DAPC has important roles in stabilizing sarcolemma and transmitting force generated in the muscle sarcomere to the ECM (Petrof et al. 1993).
In order to elucidate if the unique anatomy, morphology and function of the palate muscles are also reflected in its muscle fiber cytoarchitecture, cytoskeletal proteins in two human palate muscles were investigated and the result compared with findings in the limb muscles.
Materials and methods
Approval of the study
The regional Medical Ethical Committee in Umeå (Dnr 05‐130M) and the National Board of Health and Welfare, Stockholm, Sweden approved the study. The muscle samples were collected in agreement with the Declaration of Helsinki.
Muscle samples
Samples from UV and PP were taken post mortem from five previously healthy adult subjects (two males, three females, mean age 54 years, age range 46–75 years), and by biopsy procedure from five adult male subjects (mean age 40 years, range 31–51 years). The samples were taken at the base of UV and in the anterior–superior region of the PP arch. All biopsies were obtained from healthy non‐snoring subjects with normal oropharyngeal function proven by videofluoroscopic examination of swallowing function. Absence of sleep‐disordered breathing was confirmed by overnight sleep registration (Somnologica PSG software, Embla, Broomfield, CO, USA). All the 10 subjects, except one, had normal body constitution according to body mass index (< 30 kg m−2). None of them was under medical treatment, and there was no history of any significant disease, alcohol or drug abuse. In addition, autopsies were taken from two male infants (age 4 months and 1.4 years). For comparison, muscle samples from six healthy voluntary adult subjects were taken from a thigh muscle, musculus vastus lateralis, and an arm muscle, musculus biceps brachii. These two limb muscles are known to have a mixed population of fibers expressing myosin heavy chain (MyHC)I or MyHCII. Their morphology, muscle fiber type composition and cytoskeletal build up are well studied and established.
The muscle samples were mounted for transverse sectioning in OCT compound (Tissue Tek, Miles, Elkhart, IN, USA), rapidly frozen in liquid propane chilled with liquid nitrogen and stored at −80 °C until further processing. Some samples were fixed before freezing using 4% formaldehyde in 0.1 m phosphate buffer, pH 7.0, for 24 h at 4 °C. The samples were then washed overnight at 4 °C in Tyrodes solution containing 10% sucrose.
Immunohistochemistry
Serial muscle cross‐sections, 5 μm thick, were cut in a cryostat at −20 °C and mounted on glass slides. Immunohistochemical staining was performed using modified standard techniques and well‐characterized monoclonal (mAb) and polyclonal (pAb) antibodies (Abs). In brief, the sections were immersed in 5% normal non‐immune donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 15 min and rinsed in 0.01 m phosphate‐buffered saline (PBS) for 3 × 5 min. The sections were then incubated with the primary antibody diluted to appropriate concentrations in PBS with bovine serum albumin in a humid environment. Double‐ or triple‐staining procedures were performed in different combinations with different Abs. Incubation was carried out overnight at 4 °C. After additional washes in PBS, sections were incubated with the secondary Ab (37 °C for 30 min) and washed in PBS for 3 × 5 min. Bound primary Abs were visualized by indirect immunofluorescence using affinity‐purified Abs prepared for multiple labeling and conjugated with fluorochrome with different emission spectra; fluorescein (FITC), Rhodamine Red‐X (RRX; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), Alexa fluor 488 and Alexa fluor 647 (Invitrogen, CA, USA). The sections were thereafter washed in PBS for 3 × 5 min and then mounted in Vectashield Mounting Medium (H‐1000) or Mounting Medium with 4′,6‐diamidino‐2‐phenylindole (DAPI) for staining of nuclei (Vector Laboratories, Burlingame, CA, USA). Control sections were treated as above, except that the primary Abs were exchanged with non‐immune serum (for details, see Lindström & Thornell, 2009).
Abs and ligands
A panel of Abs was used for visualization of cytoskeletal‐ and membrane‐associated proteins (Table 1). For detection of dystrophin, one anti‐dystrophin mAb directed against the rod, two against the C‐terminus and one against the N‐terminus were used. Desmin was identified with three different anti‐desmin Abs. Basement membrane zone was identified with an anti‐laminin antibody. Members of DAPC were detected with mAbs against β‐dystroglycan, nNOS and caveolin‐3. In addition, membrane proteins were investigated with mAbs against spectrin and vinculin. Cytoskeletal proteins expressed during development and regeneration processes were identified by mAbs against vimentin, H‐nestin and utrophin (Blake et al. 1996; Herrmann & Aebi, 2000). A cytoskeletal protein linking actin to the Z disc of the sarcomere was visualized with an antibody directed against α‐actinin. Rhodamine Phalloidin, a ligand (R‐415; Molecular Probes, Leiden, the Netherlands) conjugated to the red‐orange fluorescent dye, tetramethylrhodamine (TRITC), was used to identify filamentous actin (F‐actin).
Table 1.
Antibodies used for immunohistochemistry
| Antibody | Product code | Specificity | Genea | Host/clone | Dilution | Source |
|---|---|---|---|---|---|---|
| Dystrophin | GTX15277 | Human dystrophin (C‐terminus) | DMD | pAb‐rabbit | 1 : 7500 | 1 |
| Dystrophin | NCL‐DYS1 | Human dystrophin (rod domain) | DMD | mAb‐mouse/Dy4/6D3 | 1 : 5 | 2 |
| Dystrophin | NCL‐DYS2 | Human dystrophin (C‐terminus) | DMD | mAb‐mouse/Dy8/6C5 | 1 : 10 | 2 |
| Dystrophin | NCL‐DYS3 | Human dystrophin (N‐ terminus) | DMD | mAb‐mouse/DY10/12B2 | 1 : 10 | 2 |
| Desmin | M0760 | Human and animal desmin | DES | mAb‐mouse/D33 | 1 : 100 | 3 |
| Desmin | 18‐0016 | Human desmin | DES | mAb‐mouse/ZC18 | 1 : 1000 | 4 |
| Desmin | ab15200 | Human and animal desmin | DES | pAb‐rabbit | 1 : 2000 | 5 |
| Laminin | PC 128 | Human laminin | LAM | pAb‐sheep | 1 : 15000 | 6 |
| βeta‐Dystroglycan | NCL‐b‐DG | Human beta‐dystroglycan | DAG1 | mAb‐mouse/43DAG1/8D5 | 1 : 500 | 2 |
| Caveolin‐3 | 610421 | Animal caveolin‐3 | CAV3 | mAb‐mouse/26/caveolin‐3 | 1 : 500 | 7 |
| nNOS | AB5380 | Human and animal nNOS, NOS‐I, bNOS | NOS1 | pAb‐rabbit | 1 : 5000 | 8 |
| Vinculin | V9131 | Human vinculin | VCL | mAb‐mouse/hVIN‐1 | 1 : 5000 | 9 |
| H‐Nestin | MAB1259 | Human nestin | NES | mAb‐mouse/196908 | 1 : 250 | 10 |
| Utrophin | sc‐33700 | Human utrophin | UTRN | mAb‐mouse/8A4 | 1 : 200 | 11 |
| α‐Actinin | ab9465 | Human and animal α‐actinin (sarcomeric) | ACTN3 | mAb‐mouse/EA‐53 | 1 : 5000 | 9 |
| Spectrin | NCL‐SPEC2 | Human and animal spectrin | SPBT | mAb‐mouse/RBC1/5B1 | 1 : 1000 | 12 |
| Vimentin | sc‐6260 | Human vimentin | VIM | mAb‐mouse/V9 | 1 : 250 | 11 |
| MyHC | A4.840 | MyHCI | MYH7 | mAb‐mouse | 1 : 20 | 13 |
| MyHC | N2.261 | MyHCI | MYH7 | mAb‐mouse | 1 : 400 | 13 |
| MyHCIIa | MYH2 | |||||
| MyHCeom | MYH13 | |||||
| MyHCα‐cardiac | MYH6 | |||||
| MyHC | MyHC‐slow tonic | MYH14 | pAb‐rabbit | 1 : 500 | 14 |
Official gene nomenclature according to OMIM (http://www.ncbi.nlm.nih.gov/omim/).
1. GeneTex, Taiwan; 2. Novocastra Laboratories, UK; 3. Dako, Sweden; 4. Invitrogen, CA, USA; 5. Abcam, UK; 6. Binding Site, USA; 7. BD Biosciences, USA; 8 Chemicon, CA, USA; 9. Sigma‐Aldrich, UK; 10. R&D Systems, UK; 11. Santa Cruz Biotechnology, UK; 12. Leica Biosystems, UK; 13. Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Biological Sciences, Iowa City, Iowa, USA; 14. gift from Stefano Schiaffino, CNR Inst of Neuroscience, Padova, Italy.
DMD, Duchenne muscular dystrophy; mAb, monoclonal antibody; nNOS, neuronal nitric oxide synthase; pAB, polyclonal antibody.
For fiber phenotype classification, muscle cross‐sections were stained with two mAbs against the major contractile MyHC isoforms (mAb A4.840, strong affinity for MyHCI, mAb N2.261 strong affinity to MyHCIIa, weak affinity to MyHCI, no affinity to MyHCIIx). Slow tonic MyHC was identified with mAb MYH14.
Muscle fiber classification
Based on the immunostaining pattern for the different MyHC mAbs, the muscle fibers in the UV and PP were classified as slow MyHCI or fast MyHCII.
Morphometric analysis
Four random and four selected areas of each muscle cross‐section were scanned at 20 × magnification with a fluorescence microscope (Leica DM6000B; Leica Microsystems CMS GmbH, Wetzlar, Germany) equipped with a color CCD camera (Leica DFC490) and a digital high‐speed fluorescence CCD camera (Leica DFC360 FX). The random areas were obtained from the entire muscle cross‐section, while the selected areas were chosen from areas showing a high presence of muscle fibers with low or absence of desmin and dystrophin staining. The total number of stained or weakly/unstained fibers was quantified manually on each photo. The proportions of stained or weakly/unstained fibers in the random respective and chosen areas were analyzed separately. The quantification was based on 3453 muscle fibers in UV and 2920 in PP. The samples from infants were not included in the quantification of fibers immunostained for desmin and dystrophin.
Results
Comparison of staining pattern between autopsies and biopsies
The results revealed a similar staining pattern for the Abs used in autopsies and biopsies, as well as in unfixed and fixed samples, with the exception that staining intensity was slightly stronger in biopsies and fixed samples than in autopsies and unfixed samples. All sections where the primary Abs were exchanged with non‐immune serum were unstained.
Muscle fiber phenotype composition
The adult UV and PP muscles from healthy subjects were predominated by fibers containing fast MyHCII (78.4.5 ± 8.3% and 73.3 ± 6.6%, respectively). The percentage of muscle fiber phenotypes was obtained from the specific areas selected for quantification of cytoskeletal proteins. No muscle fibers were stained for mAb MYH14 against slow tonic MyHC. The two limb muscles, musculus vastus lateralis and biceps brachii, contained a checkerboard pattern of fibers mainly expressing either slow MyHCI or fast MyHCII. Both limb muscles had a slight predominance of MyHCII fibers as previously reported by the current authors and other investigators (Klitgaard et al. 1990; Staron et al. 2000; Pontén & Stål, 2007).
Cytoskeletal proteins
The immunohistochemical staining pattern for desmin, the C‐terminal of dystrophin and the DAPC members beta‐dystroglycan, nNOS and caveolin‐3 differed between palate and limb muscles (Table 2). While all fibers in limb muscles showed immunoreaction for the Abs against these proteins, a subpopulation of muscle fibers was unstained or weakly stained in the palate muscles of both adults and infants (Figs 1 and 2). Fibers unstained or weakly stained for desmin and the dystrophin C‐terminus were often abundant in certain areas of the muscle cross‐section. In the text, these fibers are designated as desmin negative (Des‐Neg) and dystrophin C‐terminal negative (DysC‐Neg) fibers, respectively, while fibers positively stained are referred to as desmin positive (Des‐Pos) and dystrophin C‐terminal positive (DysC‐Pos) fibers (Table 3). The mAbs against α‐actinin, spectrin, vinculin and the ligand rhodamine phalloidine against F‐actin showed a distinct staining pattern in all palate and limb muscles. Immunoreaction for vimentin and H‐nestin was not observed in any muscle samples. All the palate and limb muscles showed a distinct staining for the membrane protein laminin (Table 2).
Table 2.
Immunostaining of the different antibodies in adult human soft palate and limb muscles.
| Antibody | Product code | Palate muscles | Limb muscles |
|---|---|---|---|
| Dystrophin | GTX15277 | −/+ | + |
| Dystrophin | NCL‐DYS1 | + | + |
| Dystrophin | NCL‐DYS2 | −/+ | + |
| Dystrophin | NCL‐DYS3 | + | + |
| Desmin | M0760 | −/+ | + |
| Desmin | 18‐0016 | −/+ | + |
| Desmin | ab15200 | −/+ | + |
| Laminin | PC 128 | + | + |
| β‐Dystroglycan | NCL‐b‐DG | −/+ | + |
| Caveolin‐3 | 610421 | −/+ | + |
| nNOS | AB5380 | −/+ | + |
| Vinculin | V9131 | + | + |
| H‐Nestin | MAB1259 | − | − |
| Utrophin | sc‐33700 | − | − |
| α‐Actinin | ab9465 | + | + |
| Spectrin | NCL‐SPEC2 | + | + |
| Vimentin | sc‐6260 | − | − |
Positive immunoreaction in fibers/fiber membranes are marked (+) and absent staining are marked (−).
nNOS, neuronal nitric oxide synthase.
Figure 1.
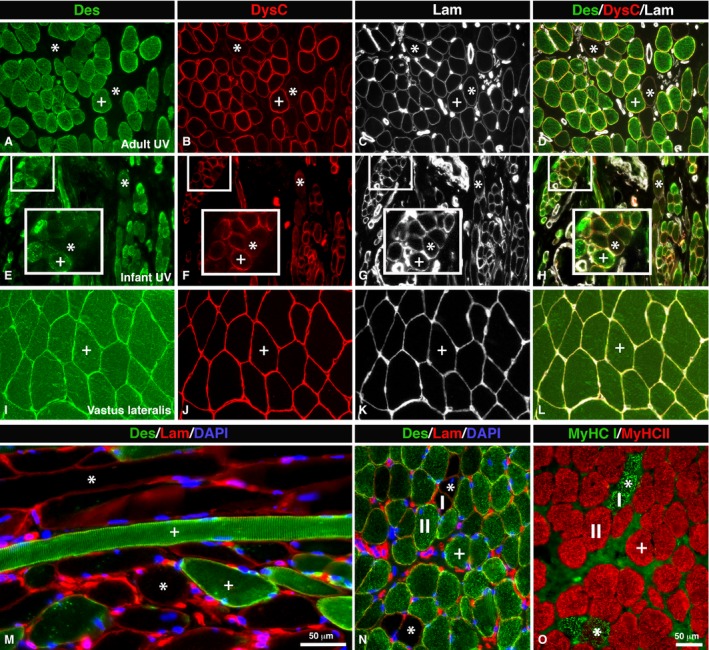
Des‐Neg and DysC‐Neg fibers in UV. Muscle sections from an adult (A–D, M–O) and infant (E–H) UV muscle and a limb muscle, musculus vastus lateralis (I–L). The sections are immunostained for desmin (Des, green color; A,E,I), the C‐terminal of dystrophin (DysC, red color; B,F,J) and laminin (Lam, white color; C,G,K). (D,H,L) and (M–O) show merged stainings. Sections (M) and (N) (Des, green color; Lam, red color) are also stained for DAPI (nucleus stained blue). (M) shows longitudinal sectioned muscle fibers double‐stained for desmin (green color) and laminin (red color). Des‐Neg/DysC‐Neg fibers are marked (*) and Des‐Pos/DysC‐Pos are marked (+). Insets from (E–H) highlight magnified Des‐Neg/DysC‐Neg fibers in UV from an infant (4 months old). Note the low or absent staining for desmin and dystrophin C‐terminal (*), but distinct staining for laminin in the sections from both the adult and infant UV muscle, while in the limb muscle all sections are positively stained. The staining pattern for contractile slow MyHCI (I, green color) and fast MyHCII (II, red color) proteins is shown in (O), and the relation to Des‐Neg/DysC‐Neg fibers is shown in (N). Notice the typical expression of slow MyHCI in Des‐Neg/DysC‐Neg fibers (stars). Scale bar: 50 μm.
Figure 2.
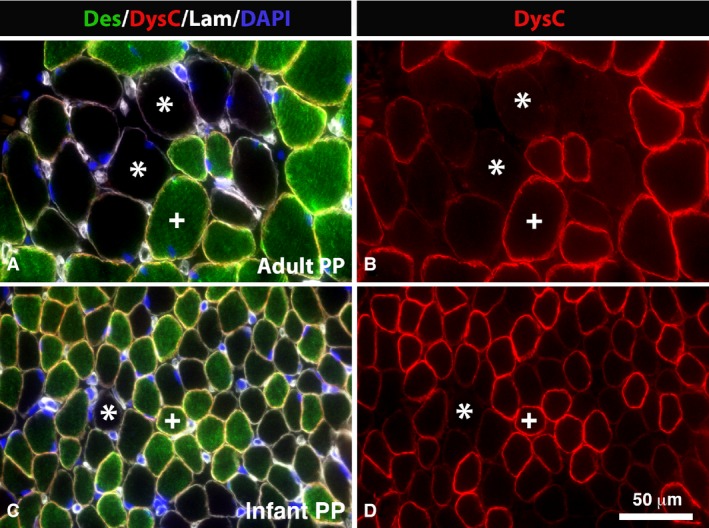
Des‐Neg and DysC‐Neg fibers in PP. Muscle cross‐sections from an adult (A,B) and infant (C,D) PP muscle multi‐stained for desmin (Des, green color), dystrophin C‐terminal (DysC, red color), laminin (Lam, white color) and DAPI (nuclei, blue color) (A,C) and just for dystrophin C‐terminal (DysC, red color; B,D). Des‐Neg/DysC‐Neg fibers are marked (*) and Des‐Pos/DysC‐Pos are marked (+). Note the high frequency of fibers unstained or weakly stained for both the dystrophin C‐terminal and desmin in adult and infant muscles. Scale bar: 50 μm.
Table 3.
Percentage (%) of of Des‐Neg, Des‐Pos, DysC‐Neg and DysC‐Pos fibers in UV and PP.
| Muscle | Random areas | Specific areas | ||
|---|---|---|---|---|
| % | SD | % | SD | |
| UV (n = 3453 muscle fibers) | ||||
| Des‐Pos, DysC‐Pos | 86.8 | 12.5 | 64.9 | 26.3 |
| Des‐Neg, DysC‐Neg | 2.7 | 2.3 | 20.4 | 26.7 |
| Des‐Neg, DysC‐Pos | 9.0 | 12.9 | 8.3 | 8.7 |
| Des‐Pos, DysC‐Neg | 1.5 | 1.0 | 6.4 | 8.5 |
| PP (n = 2920 muscle fibers) | ||||
| Des‐Pos, DysC‐Pos | 88.8 | 3.7 | 60.4 | 31.3 |
| Des‐Neg, DysC‐Neg | 5.0 | 3.2 | 17.1 | 19.7 |
| Des‐Neg, DysC‐Pos | 4.8 | 3.7 | 15.7 | 17.9 |
| Des‐Pos, DysC‐Neg | 1.4 | 0.4 | 6.8 | 7.5 |
The mean percentage values are obtained from random areas of the entire muscle cross‐section of each sample, and from selected areas with an abundance of Des‐Neg and DysC‐Neg fibers, respectively. The quantitative analysis is based on adult samples from normal subjects.
Des‐Neg, desmin negative; Des‐Pos, desmin positive; DysC‐Neg, dystrophin C‐terminal negative; DysC‐Pos, dystrophin C‐terminal positive; PP, musculus palatopharyngeus; UV, musculus uvula.
Des‐Neg fibers
In the UV muscle 11.7% of the fibers were Des‐Neg and the corresponding value in the PP muscle was 9.8% (Fig. 1; Table 2). There was a strong correlation between Des‐Neg fibers and fibers expressing MyHCI in both PP and UV. Des‐Neg fibers expressed predominately slow contractile protein MyHCI (UV 62.8 ± 22.9%, PP 74.4 ± 16.2%; Fig. 1).
DysC‐Neg fibers
In both PP and UV, a subpopulation of fibers showed weak or absence of immunoreaction for the two Abs directed against the dystrophin C‐terminus (Figs 1 and 3; Table 2). In contrast, the Abs directed against the rod and the N‐terminus showed a distinct immunoreaction (Fig. 3). In UV, 4.2% of the fibers were DysC‐Neg and in PP the corresponding value was 6.4%. DysC‐Neg fibers expressing MyHCI was 69.9 ± 21.1% in UV and 76.4 ± 6.5% in PP (Fig. 1).
Figure 3.
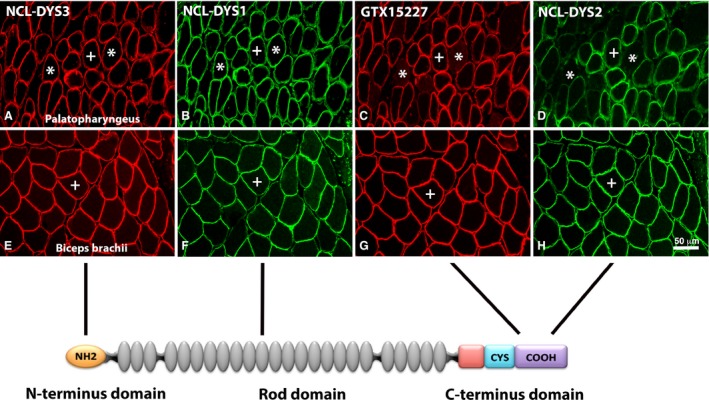
Immunoreaction of antibodies against different domains of the dystrophin molecule in palate and limb muscles. Serial cross‐sections from palatopharyngeus (A–D) and biceps brachii (E–H) stained with antibodies directed against the N‐terminal (NCL‐DYS3, red color; A,E), the rod (NCL‐DYS1, green color; B,F) and the C‐terminal (GTX15227, red color; C,G and NCL‐DYS2, green color; D,H) domains of the dystrophin molecule. The schematic sketch shows domains of the dystrophin molecule corresponding to antibody affinity. Note that while all domain‐specific antibodies showed immunoreaction in the limb muscle, a subgroup of fibers was unstained or weakly stained for the two antibodies directed against the C‐terminus in the UV (asterisks). Muscle fibers stained for all four antibodies are marked (+). Scale bar: 50 μm.
Correlation between Des‐Neg and DysC‐Neg fibers
The majority of muscle fibers lacking immunoreaction for the dystrophin C‐terminus also lacked immunoreaction for desmin. The proportion of fibers classified as both DysC‐Neg and Des‐Neg was 2.7% in UV and 5.0% in PP (Figs 1 and 2; Table 3).
DAPC
In limb muscles, the mAbs against caveolin‐3, β‐dystroglycan and nNOS showed a strong immunoreaction in the membranes of the fibers, while in palate muscles the staining pattern varied from absent to strong (Table 2). In the palate muscles, the immunoreaction for caveolin‐3 and β‐dystroglycan were commonly strong in the membrane of Des‐Neg/DysC‐Neg fibers, whereas the immunoreaction for nNOS was weak or absent. Des‐Pos/DysC‐Pos fibers showed in general a moderate membrane staining reaction for caveolin‐3, β‐dystroglycan and nNOS, but there was a variation in staining pattern from absent to strong. Moreover, an intramyofibrillar staining was also observed in palate muscles for caveolin‐3, β‐dystroglycan and nNOS that largely correlated to muscle fiber phenotype; fibers expressing MyHCI were commonly unstained or weakly stained, whereas fibers expressing MyHCII were weak to moderately stained. Limb muscle fibers generally lacked any distinct intramyofibrillar reaction for these Abs.
Discussion
The present study shows that the muscles of the human soft palate differ from limb muscles in their expression of cytoskeletal proteins. The most interesting finding was a low or absent immune response in a subgroup of muscle fibers for the Abs against the IF desmin and the C‐terminal of dystrophin, while all fibers in limb muscles expressed a distinct immune response for these Abs (Figs 1, 2, 3). In human muscles, abnormal expression or substitute isoforms of these ubiquitous cytoskeletal muscle proteins has only been reported in genetic disorders.
The lack of firm muscle attachments in both UV and PP suggest low capacity to develop static contractions and high tension. This anatomy together with the marked predominance of MyHCII fibers reflects the capacity of these muscles to perform very fast contractions. In contrast, the elevating muscles of the soft palate are predominated by MyHCI fibers (Stål & Lindman, 2000), indicating capacity for slower contractions and more sustained positioning of the soft palate. The specific fiber type composition together with an atypical cytoarchitecture suggests that each of the palate muscles has evolved in a unique way to meet complex demands in oropharyngeal functions.
Force generation of the muscle sarcomeres is transmitted to sarcolemma and ECM by a complex network and interaction of cytoskeletal proteins. An important cytoskeletal protein required for this interaction is IF desmin. This protein is regarded to be ubiquitous in skeletal muscles. However, desmin has recently been questioned as a normal component in fibers of extraocular muscles. Janbaz et al. (2014) reported a low or absent expression of desmin in a subpopulation of muscle fibers expressing slow tonic MyHC and/or slow MyHCI. Here it is shown that Des‐Neg fibers preferentially express slow MyHCI also in the soft palate muscles suggesting phenotypic specialization. The atypical cytoskeletal architecture in a subpopulation of fibers in palate and extraocular muscles, but not in limb muscles, provides further evidence that the fiber composition in cranial muscles is more complex than in limb muscles (Stål et al. 1987, 1990, 1994, 1995, 2003; Klitgaard et al. 1990; Staron et al. 2000; Kjellgren et al. 2006; Pontén & Stål, 2007; Janbaz et al. 2014).
Another novel finding was the absence or low immunoreaction for the C‐terminal domain (–COOH) of the dystrophin molecule in subpopulation of UV and PP muscle fibers, while the N‐terminal (–NH2) and rod domain of the molecule showed a distinct immunoreaction in all fibers (Fig. 3; Tables 1 and 2). In the UV muscle approximately 4% of all fibers were unstained or weakly stained for the Abs directed against the dystrophin C‐terminal, and the corresponding value in the PP was approximately 6% (Table 3). Interestingly, most of the fibers unreactive for Abs against the dystrophin C‐terminal were also unreactive for the Abs against nNOS and IF desmin, while the staining for β‐dystroglycan and caveolin 3 was generally strong in the membrane. Notably, these proteins are all linked to the C‐terminus domain of the dystrophin molecule. This might reflect a unique molecular linkage between desmin and dystrophin and its associated membrane protein complex in palate muscles. Consequently, this subgroup of fibers may have special biomechanical properties, i.e. influencing fiber stiffness, sarcolemmal deformability, stability of costameres, contraction velocity and transmission of forces to the ECM.
In Duchenne muscular dystrophy (DMD), a progressive lethal muscle‐wasting disease caused by mutations in genes coding for dystrophin, there is an upregulation of utrophin. Utrophin, a paralog to dystrophin in muscles, is normally expressed on the sarcolemma of developing and regenerating fibers, but is ultimately replaced by dystrophin of normal maturing fibers (Love et al. 1989). No upregulation of utrophin was found as a substitute in DysC‐Neg fibers. Moreover, in DMD, loss of dystrophin results in reduction of the entire DAPC, leaving the membrane highly susceptible to contraction‐induced injury and hypoxic stress (Greenberg et al. 1994; Banks et al. 2014). This scenario is highly unlikely for the DysC‐Neg fibers in normal human palate muscles.
The absent or low immunoreaction for desmin and dystrophin C‐terminus and the unusual staining for DAPC in both immediately frozen biopsies and in autopsies of adult and infant palate muscles strongly point towards a unique cytoarchitecture rather than muscle fiber abnormalities. The otherwise well‐preserved cytoskeletal architecture visualized by the distinct staining for laminin and the organized labeling of the myofibrils for other ubiquitous cytoskeletal and myofibrillar proteins such as α‐actinin and F‐actin confirms that these fibers were intact and healthy.
Conclusion
The novel findings of a unique cytoarchitecture in a subgroup of normal soft palate muscle fibers most likely reflect specialization of the muscles to meet complex requirements in intricate oropharyngeal functions, such as breathing, oral communication and swallowing. These findings also challenge the present concept of desmin and other cytoskeletal proteins as ubiquitous components in cranial muscles. This must be taken into account while assessing pathology in oropharyngeal muscles affected by injury, such as vibratory/stretch damage in sleep‐disordered breathing, or neuromuscular disorders.
Author contributions
Farhan Shah performed experiments, evaluated the data and prepared the manuscript. Diana Berggren acquired biopsies, evaluated the data and prepared the manuscript. Thorbjörn Holmlund acquired biopsies, evaluated the data and reviewed the manuscript. Eva Levring Jäghagen evaluated the data and reviewed the manuscript. Per Stål was the PI, supervised experiments, data evaluation and manuscript preparation.
Acknowledgements
This work was supported by the Heart‐Lung Foundation, Sweden (nr 20110210, 20140339) and Insamlingsstiftelsen, Umeå University, Sweden (Dnr 223‐1881‐13). The authors thank Professor Lars‐Eric Thornell for valuable inputs and comments, Mrs Anna‐Karin Olofsson for excellent technical assistance, Dr Nausheen Khan for helpful comments, and Dr Gustav Andersson for his contribution of the sketch in Fig. 3. There is no conflict of interest.
References
- Banks GB, Combs AC, Odom GL, et al. (2014) Muscle structure influences utrophin expression in mdx mice. PLoS Genet 10, e1004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DJ, Tinsley JM, Davies KE (1996) Utrophin: a structural and functional comparison to dystrophin. Brain Pathol 6, 37–47. [DOI] [PubMed] [Google Scholar]
- Campbell KP (1995) Three muscular dystrophies: loss of cytoskeleton‐extracellular matrix linkage. Cell 80, 675–679. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y, Bloch R, Kouloumenta A, et al. (2007) Muscle intermediate filaments and their links to membranes and membranous organelles. Exp Cell Res 313, 2063–2076. [DOI] [PubMed] [Google Scholar]
- Davies KE, Nowak KJ (2006) Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol 7, 762–773. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63, 345–382. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Sunada Y, Campbell KP, et al. (1994) Exogenous Dp71 restores the levels of dystrophin associated proteins but does not alleviate muscle damage in mdx mice. Nat Genet 8, 340–344. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U (2000) Intermediate filaments and their associates: multi‐talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 12, 79–90. [DOI] [PubMed] [Google Scholar]
- Janbaz AH, Lindström M, Liu JX, et al. (2014) Intermediate filaments in the human extraocular muscles. Invest Ophthalmol Vis Sci 55, 5151–5159. [DOI] [PubMed] [Google Scholar]
- Kjellgren D, Stål P, Larsson L, et al. (2006) Uncoordinated expression of myosin heavy chains and myosin‐binding protein C isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 47, 4188–4193. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, et al. (1990) Ageing alters the myosin heavy chain composition of single fibers from human skeletal muscle. Acta Physiol Scand 140, 55–62. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Kahane JC (1990) Histologic study of the normal human adult soft palate. Cleft Palate J 27, 26–34. [DOI] [PubMed] [Google Scholar]
- Lindström M, Thornell LE (2009) New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power‐lifters and sedentary men. Histochem Cell Biol 132, 141–157. [DOI] [PubMed] [Google Scholar]
- Love DR, Hill DF, Dickson G, et al. (1989) An autosomal transcript in skeletal muscle with homology to dystrophin. Nature 339, 55–58. [DOI] [PubMed] [Google Scholar]
- Paulin D, Li Z (2004) Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301, 1–7. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Stedman HH, Shrager JB, et al. (1993) Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol 265, C834–C841. [DOI] [PubMed] [Google Scholar]
- Pontén EM, Stål PS (2007) Decreased capillarization and a shift to fast myosin heavy chain IIx in the biceps brachii muscle from young adults with spastic paresis. J Neurol Sci 253, 25–33. [DOI] [PubMed] [Google Scholar]
- Shah SB, Davis J, Weisleder N, et al. (2004) Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J 86, 2993–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Fürst DO, Thornell LE (1992) The cytoskeletal lattice of muscle cells. Eur J Biochem 208, 559–572. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Yu S (2006) Stretching to meet needs: integrin‐linked kinase and the cardiac pump. Genes Dev 20, 2327–2331. [DOI] [PubMed] [Google Scholar]
- Stål PS, Lindman R (2000) Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat 197, 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stål P, Eriksson PO, Eriksson A, et al. (1987) Enzyme‐histochemical differences in fibre‐type between the human major and minor zygomatic and the first dorsal interosseus muscles. Arch Oral Biol 32, 833–841. [DOI] [PubMed] [Google Scholar]
- Stål P, Eriksson PO, Eriksson A, et al. (1990) Enzyme‐histochemical and morphological characteristics of muscle fibre types in the human buccinator and orbicularis oris. Arch Oral Biol 35, 449–458. [DOI] [PubMed] [Google Scholar]
- Stål P, Eriksson PO, Schiaffino S, et al. (1994) Differences in myosin composition between human oro‐facial, masticatory and limb muscles: enzyme‐, immunohisto‐ and biochemical studies. J Muscle Res Cell Motil 15, 517–534. [DOI] [PubMed] [Google Scholar]
- Stål P, Eriksson PO, Thornell LE (1995) Muscle‐specific enzyme activity patterns of the capillary bed of human oro‐facial, masticatory and limb muscles. Histochem Cell Biol 104, 47–54. [DOI] [PubMed] [Google Scholar]
- Stål P, Marklund S, Thornell LE, et al. (2003) Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs 173, 147–161. [DOI] [PubMed] [Google Scholar]
- Staron RS, Hagerman FC, Hikida RS, et al. (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48, 623–629. [DOI] [PubMed] [Google Scholar]


