Abstract
The zebrafish is as an important vertebrate animal model system for studying developmental processes, gene functions and signalling pathways. It is also used as a model system for the understanding of human developmental diseases including those related to the skeleton. However, surprisingly little is known about normal zebrafish skeletogenesis and osteogenesis. As in most vertebrates, it is commonly known that the bones of adult zebrafish are cellular unlike that of some other teleosts. After careful histological analyses of each zebrafish adult bone, we identified several acellular bones, with no entrapped osteocytes in addition to several cellular bones. We show that both cellular and acellular bones can even occur within the same skeletal element and transitions between these two cell types can be found. Furthermore, we describe two types of osteoblast clusters during skeletogenesis and two different types of endochondral ossification. The epiphyseal plate, for example, lacks a zone of calcification and a degradation zone with osteoblasts. A new bone type that we term tubular bone was also identified. This bone is completely filled with adipose tissue, unlike spongy bones. This study provides important insight on how osteogenesis takes place in zebrafish, and especially on the transition from cellular to acellular bones. Overall, this study leads to a deeper understanding of the functional histological composition of adult zebrafish bones.
Keywords: acellular, bone, Danio rerio, ossification, skeletogenesis, skeleton
Introduction
Vertebrate bones develop through two main mechanisms, intramembranous and endochondral bone formation. During intramembranous ossification, mesenchymal cells condense and differentiate directly into osteoblasts. In contrast, in endochondral ossification, mesenchymal cells condense and differentiate into chondrocytes of cartilages. During perichondral ossification, bone development starts peripheral to a cartilage beneath the perichondrium. In both perichondral and endochondral ossification, the cartilages are the template for the future bones and with further development they are replaced by bone. Perichondral ossification has been viewed by some as a form of endochondral ossification (as cartilage is replaced by bone) and by others as a form of intramembranous ossification (as it is within the perichondrium that osteoblasts differentiate and lay down the bone collar) (Hall, 2015). A classic example of this is the ossification of Meckel's cartilage. Little attention, however, has been given to how osteoblast deposition of bone matrix contributes to increasing the size of these bones (Franz‐Odendaal, 2011).
The zebrafish has become an important model system to study bone formation because similar genes and cells make the skeleton in this vertebrate (as compared to humans). Due to the life‐long growth of fish, the process of intramembranous and endochondral ossification continues into adulthood and can occur in combination with skeletal remodelling by osteoclasts. However, surprisingly little is known about the adult zebrafish skeleton, hence most publications deal with very early bone development. Therefore, the processes of intramembranous and endochondral ossification have not been studied in detail in adult zebrafish until now.
In teleost fish, two bone types have been identified: Cellular bones with entrapped osteocytes (similar to mammals and reptiles) and acellular bones without osteocytes (von Kölliker, 1859; Moss, 1961a; Ekanayake & Hall, 1987; Meunier, 1989; Meunier & Huysseune, 1992; Huysseune, 2000; Witten et al. 2004; Witten & Huysseune, 2009). Zebrafish bones have been previously described as cellular (Witten & Huysseune, 2009; Cao et al. 2011). However, in several more recent publications, and especially relating to the dermal bony fin rays, the bones appear to be acellular (Murciano et al. 2002; Laue et al. 2011; Akiva et al. 2015). The differences in the existing literature, might be due to the analysis of specific bones only in each of these studies. Therefore, we reinvestigated the histological constitution of all adult zebrafish bones and importantly we show that both cellular and acellular bones occur equally, and are distributed over the entire zebrafish skeleton. A further goal of this study was to clarify the occurrence and comparative histology of different bone types in the adult zebrafish, such as compact bones, spongy bones and chondroid bones. We also identify a new bone type, which originates exclusively from a special mode of endochondral ossification, which we term tubular bone.
Materials and methods
Animals
Adult zebrafish (Danio rerio) were used for all experiments and were raised in the Mount Saint Vincent University (Canada) fish facility according to standard procedures (28.5 °C, 12‐h light cycle). All protocols follow the Canadian Council on Animal Care guidelines and were approved annually by the SMU‐MSVU Animal Care committee (Canada).
Histology
Six adults were anesthetized using 0.1% MS222, and then fixed in 4% paraformaldehyde overnight at 4 °C. Fish were decalcified and embedded in low melting point paraffin wax according to standard procedures. Fish were embedded in various orientations to enable examination of ossification throughout the skeleton. Sections were cut 6 μm thick on a Leitz microtome 1512 (Wetzlar, Germany). Sections were stained with either Mallory's Trichrome staining or Hall Brunt's Quadruple staining to visualise cartilage, osteoid and mineralised bone clearly (Flint & Lyons, 1975; Hall, 1986).
Results
Our analysis below is based on an extensive analysis of serial sections of the adult zebrafish skeleton. Here we describe our general observations and summarize the cell clusters and bone types we identified.
Osteogenesis
Intramembranous ossification
When examining the skeleton, we found matrix‐depositing osteoblasts in clusters at the end of growing bones (Fig. 1). This location of osteoblast clusters has previously been reported for teleost fish (Witten et al. 1997). Based on cell cluster size, location and nuclei shape, we could identity two different types of osteoblast clusters in zebrafish bones. Intramembranous ossification occurs via osteoblasts of type I and II.
Figure 1.
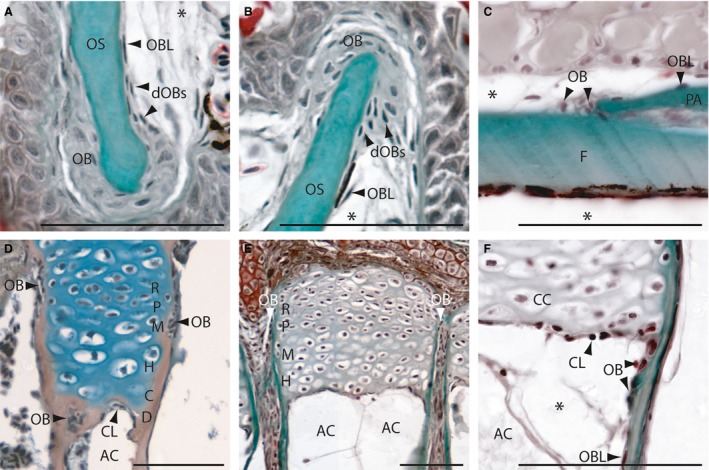
Intramembranous (A‐C) and endochondral (D‐F) ossification in zebrafish on longitudinal (A,B,E,F) and transversal (C,D) Mallory's trichrome‐stained sections. (A,B) Osteoblasts cluster type I (OB) at the anterior premaxillary (A) and dentary (B). (C) Osteoblast clusters type II at the coronal suture of the frontal and parietal bone. (D) Section of an epiphysial plate of a radial located in the pectoral girdle, showing the epiphysial growth plate of type I endochondral bones. (E,F) Sections of the epiphysial plate with type II ossification located in the hypurals of the caudal fin. (F) Detail of the active chondroclast zone. AC, adipocyte; C, zone of calcification; CC, chondrocyte; CL, chondroclasts; D, zone of cartilage degradation; dOBs, differentiating osteoblasts; F, frontal bone; H, zone of hypertrophic chondrocytes; M, zone of maturation; OB; osteoblast; OBL, osteoblast‐like cell; OS, osseus (mineralized bone); P, zone of proliferation; PA, parietal bone; R, zone of reserve cartilage. *Artefact. Scale bars: 50 μm.
Osteoblast cluster type I
Type I clusters are large clusters (composed of more than 25 cells) situated at the edges of outgrowing flat bones (Fig. 1A, B). Examples include the dentary, the maxillary and the frontal bone in the metopic suture. Here, the osteoblasts have large oval, round or irregularly shaped nuclei. Lateral to these osteoblasts is a zone of differentiating osteoblasts (dOBs; Fig. 1A,B). Within this zone, the cell size and nuclei are smaller and the cells become more and more elongated. It appears as if the cells in the type I osteoblast clusters differentiate into the typical spindle‐shaped osteoblast‐like cells of the perichondrium (Fig. 1A,B). These spindle‐shaped osteoblast‐like cells cover all zebrafish bones in a single (mono‐) layer; unlike that of the perichondrium of mammals, which is typically multi‐layered. These are the osteoblast clusters previously identified by Witten & Villwock (1997) in Oreochromis niloticus.
Osteoblast cluster type II
Type II osteoblast clusters are smaller. with only about 4–12 osteoblasts. These clusters are distributed over the entire fish skeleton and are more common than osteoblast clusters type I. They also do not contain cells with large rounded nuclei (Fig. 1C; also compare Fig. 1D, 1F, 4C, 4F and 5D), but rather, these osteoblasts are reduced in size, with slightly elongated nuclei. Therefore, they are more comparable to the osteoblasts observed in the differentiation zone of the type I clusters (compare dOB Fig. 1A, B).
Endochondral ossification
Two types of endochondral ossification were identified in the adult zebrafish skeleton.
Type I
In type I endochondral ossification, the composition of the epiphysis and the epiphysial growth plate of zebrafish is typical of that seen in other vertebrates (Fig. 1D) with a zone of reserve cartilage, a proliferation zone with columnar cartilage followed by a zone of hypertrophic chondrocytes with vesicular cartilage. In normal spongy bones, this zone is followed by a zone of calcification, in which the cartilage matrix becomes ossified and is followed by a degradation zone with chondroclasts and osteoblasts (Fig. 1D). Laterally, the epiphysial growth plate is covered by bony tissue. At the edges of these bones, type II osteoblast clusters are detectable (Fig. 1D). From these clusters, osteoblast‐like cells arise. These osteoblast‐like cells cover the outside of the endochondral bones in a mono‐layered perichondrium as described above.
Type II
Within the hyomandibular, the branchial arches, the ethmoid and the hypuralia, an unusual mode of endochondral ossification was detected that produces tubular bones (described below). The constitution of the cartilaginous epiphysial growth plate resembles that of type I endochondral ossification. However, following the zone of hypertrophic chondrocytes, a zone of calcification and a degradation zone with osteoblasts is missing (Fig. 1E). Here, a compact zone of active chondroclasts is detectable (Fig. 1E, F). This is in contrast to the type I endochondral ossification in that the hypertrophic zone is directly adjacent to adipose tissue. Based on the increased chondroclast activity observed (Fig. 1F), this results in tubular concave bones filled only with adipose tissue. Moreover, at the edges of these bones, where the active chondroclast zone connects with the bone, osteoblast clusters of type II are observed. From these clusters, the osteoblast‐like cells that cover the inside of the bones arise (Fig. 1F). The osteoblast‐like cells that cover the outside of the bone arise similarly to those in type I endochondral ossification within a typical mono‐layer (Fig. 1E and 4D).
Bone types
Macroscopically, four bone types are distinguishable: compact bones, spongy bones, tubular bones and chondroid bones (Fig. 2, Tables 1, 2, 3). However, it is important to note that these bone types are not always distinct and it appears as if there is a transition between each bone type that is fluid. Each type is discussed below.
Figure 2.
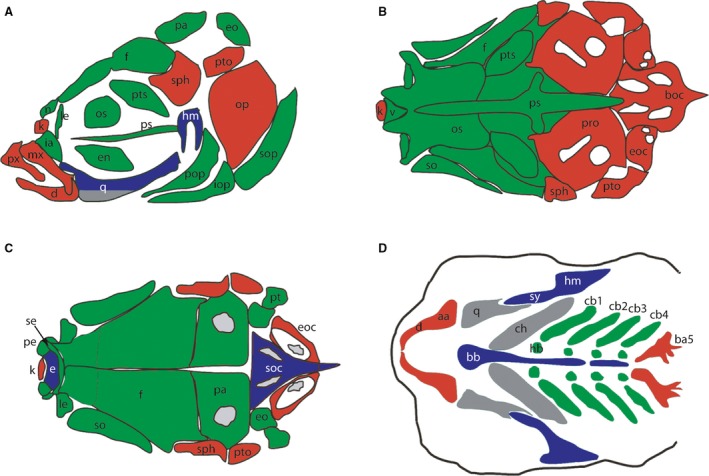
Diagrams depict the bones that are acellular compact bones (green), cellular compact bones (red), tubular bones (blue) and spongy bone (grey). (A) Side view of the bones of an adult skull. (B) Dorsal view of the dorsal aspect of the adult skull. (C) Base of the neurocranium with the pharyngeal skeleton removed. (D) Pharyngeal skeleton. aa, anguloarticular; ba5, fifth branchial arch; bb, basibranchials; boc, basioccipital; cb1‐5, ceratobranchial 1‐5; ch, ceratohyal; d, dentary; e, ethmoid; en, endopterygoid; eo, epioccipital; eoc, exoccipital; f, frontal; hb, hypobranchial; hm, hyomandibula; ia, infraorbital; iop, interopercular; k, kinethmoid; le, lateral ethmoid; mx, maxillary; n, nasal; op, opercle; os, orbitosphenoid; pa, parietal; pe, preethmoid; pop, preopercular; pro, prootic; ps, parasphenoid; pto, pterotic; px, premaxillary; pt, posttemporal; pts, pterosphenoid; q, quadrate; se, supraethmoid; so, supraorbital; sop, subopercular; sph, sphenotic; sy, sympleptic; v, vomer. Drawings were modified from Kague et al. (2012).
Table 1.
Histological composition of the bones of the dermatocranium
| Phylogenetic source | Bone | Cellular | Acellular | Compact | Spongy | Tubular |
|---|---|---|---|---|---|---|
| Fascial series | Premaxilla | + | + | |||
| Maxilla | + | + | ||||
| Nasal | + | + | ||||
| Orbital series | Infraorbital 1 | + | + | |||
| Infraorbital 2 | + | + | ||||
| Infraorbital 3 | + | + | ||||
| Infraorbital 4 | + | + | ||||
| Infraorbital 5 | + | + | ||||
| Scleral ossicles | + | + | ||||
| Supraorbital | + | + | ||||
| Temporal series | Posttemporal | + | + | |||
| Supratemporal | + | + | ||||
| Vault series | Frontal bone | + | + | |||
| Parietal | + | + | ||||
| Palatal series | Vomer | + | + | |||
| Supraethmoid | + | + | ||||
| Ectopterygoid | + | + | ||||
| Endpoterygoid | + | + | ||||
| Parasphenoid | + | + | ||||
| Mandibular series | Anguloarticular | + | + | |||
| Coronomeckelian | + | + | ||||
| Dentary | + | + | ||||
| Retroarticulara | + | + | ||||
| Opercular series | Opercular bone | + | (+) | + | ||
| Interopercular | + | + | ||||
| Subopercular | + | + | ||||
| Preopercular | + | + | ||||
| Branchiostegal rays (1–3) | + | (+) | + |
Part of the splanchnocranium.
Table 2.
Histological composition of the bones of the chondrocranium
| Phylogenetic source | Bone | Cellular | Acellular | Compact | Spongy | Tubular |
|---|---|---|---|---|---|---|
| Ethmoid series | Ethmoid | + | + | |||
| Kinethmoid | + | + | ||||
| Lateral ethmoid | + | + | ||||
| Preethmoid | + | + | ||||
| Sphenoid series | Orbitosphenoid | + | + | |||
| Pterosphenoid | + | + | ||||
| Otic series | Epioccipital | + | + | |||
| Prootic | + | + | ||||
| Pterotic | + | + | ||||
| Sphenotic | + | + | (+) | |||
| Occipital series | Basioccipital | + | + | |||
| Exoccipital | + | + | ||||
| Supraoccipital | + | + |
Table 3.
Histological composition of the bones of the splanchnocranium
| Phylogenetic source | Bone | Cellular | Acellular | Compact | Spongy | Tubular |
|---|---|---|---|---|---|---|
| Palatoquadrate arch | Metapterygoid | + | (+) | + | (+) | |
| Palatine | + | + | ||||
| Quadrate | + | + | (+) | |||
| Hyoid arch | Basiyhal | + | + | |||
| Ceratohyal | + | + | ||||
| Epihyal | + | + | ||||
| Hyomandibula | + | + | ||||
| Hypohyal (d, v) | + | + | ||||
| Symplectic | + | + | ||||
| Branchial arches | Basiobranchials | + | + | |||
| Ceratobranchials | + | + | (+) | |||
| Epibranchials | + | + | ||||
| Hypobranchialsa | + | + | ||||
| Pharyngobranchials | + | + | ||||
| Branchial arch 5 | + | + |
Part of the dermatocranium.
Compact bones
Generally, in compact bones the small osteoblast clusters (type II) are absent at the bone surfaces. All compact bones (cellular and acellular) are covered by spindle‐shaped osteoblast‐like cells with elongated cell nuclei (Fig. 3).
Figure 3.
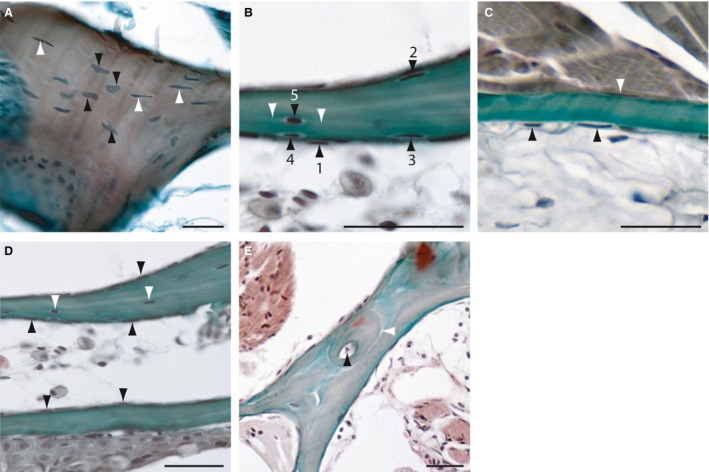
Histology of adult zebrafish compact bones on transverse Mallory's Trichrome‐stained sections. (A) Section of the cellular opercular bone. Black arrowheads are indicating round osteocytes with oval, round or atypical formed nuclei and white arrowheads elongated osteocytes with spindle‐shaped nuclei. (B) Transition from osteoblast‐like cells (1) through intermediary stages to partial bone entrapment of cells (2–4), and finally to full entrapment as osteocytes (5; black arrowheads). White arrowheads mark the cellular processes of an osteocyte. (C) Section of an acellular bone of the opercle. Arrowheads indicate osteoblast‐like cells at the surfaces. (D) Section of two branchiostegal rays with cellular (upper) and acellular (lower) parts. Black arrowheads indicate osteoblast‐like cells and white arrowheads, osteocytes. (E) Section of the lateral ethmoid with an osteon. Black arrowhead is indicating the Haversian canal and the white arrowhead marks the cement line to the surrounding bone tissue. Scale bars: 25 μm.
Cellular bones
The majority of the zebrafish skull bones are composed of cellular bones with cells that are entrapped in the bone matrix as osteocytes (Fig. 3A, Tables 1, 2, 3). Here, two osteocyte cell types are distinguishable. The first type is round with oval, round or atypical nuclei (Fig. 3A). The second type of osteocyte is smaller, spindle‐shaped and with elongated nuclei. This latter type of osteocyte was identified as the most common type found in zebrafish (Fig. 3A,B). Moreover, these cells typically have two cytoplasmic processes (Fig. 3B). This osteocyte type originates from the osteoblast‐like cells that line the bony tissue and hence have become entrapped into the bones as osteocytes (Fig. 3B). Cellular compact bone was also found in the lepidotrichia of the fins.
Acellular bones
In addition to the cellular bones, the zebrafish skeleton is also composed of several acellular bones with no entrapped osteocytes (Fig. 3C). These include the lateral ethmoid, the nasal bone, orbitosphenoid, the frontal bone, the subraorbital bone, the parietal bone, pterotic bone, the supratemporal bone and basioccipital bone. However, in some sections, single entrapped osteocytes were detectable and resemble the osteocytes of the cellular bones. Moreover, some bones have cellular and acellular parts, such as the branchiostegal rays (Fig. 3E). Therefore, we conclude that in zebrafish the transition from cellular to acellular bones is fluid (Fig. 3D). In our study, we detected acellular compact bones in the zebrafish skull (Tables 1, 2, 3) and in the vertebrae of the axial skeleton.
Osteons
Osteons were only rarely detected within the zebrafish skeleton. They are composed of a central Haversian canal and bone lamella (Fig. 3E). Moreover, osteons are sharply marked off by a cement line to the surrounding bony tissue (Fig. 3E). Importantly, osteocytes that are similar to those observed in mammals were not observed within the osteons of zebrafish.
Spongy bones
Spongy bones in zebrafish are present throughout the bony trabeculae network and arise from the process of endochondral ossification type I (Fig. 4A). The interspace in between the trabeculae is filled with adipocytes and hematopoietic connective tissue is absent; this is in contrast to mammals (Fig. 4A‐C). The inner and outer bony surfaces are covered with the osteoblast‐like cells. In the inner part, osteoblast clusters of type II, as well as osteoclasts, are observable (Fig. 4C). In our study, we detected spongy bone in the pectoral girdle, the pterosphenoid, the lower quadrate bone, the ceratohyal and the anguloarticular.
Figure 4.
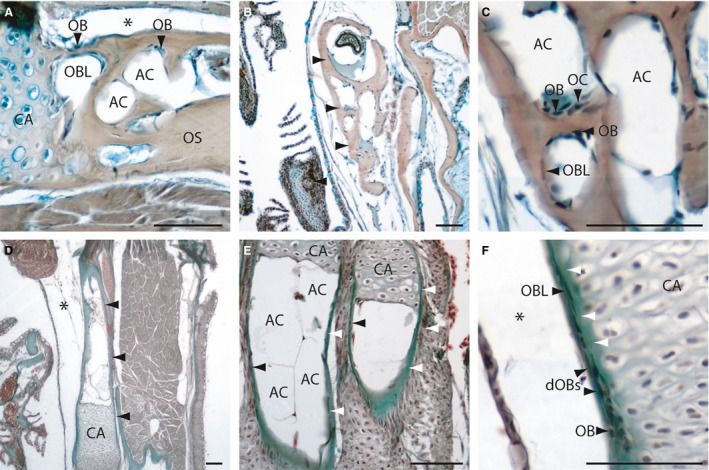
Histology of adult zebrafish spongy bones (A‐C) and tubular bones (D‐F) on transversal (A‐D, F) and longitudinal Mallory's Trichrome‐stained sections (E). (A) Section of the pectoral girdle and the ceratohyal bone (B, arrowheads). (C) Detail of a spongy bone (the ceratohyal), with osteoblasts, osteoclasts, osteoblast‐like cells and adipose cells. (D) Section of the tubular hyomandibular (arrowheads). (E) Section of two hypuralia, arrowheads are indicating the osseus. (F) Detail of the hyomandibular, with osteoblasts (bone collar cells) at the outer surface developing to osteoblast‐like cells. White arrowheads indicate the developing osseus. AC, adipocyte; CA, Cartilage; dOBs, differentiating osteoblasts; OB, osteoblast; OBL, osteoblast‐like cell; OC, osteoclast; OS, osseus. *Artefact. Scale bars: 50 μm.
Tubular bones
In tubular bones, the space inside the bones is filled with adipose tissue. This is in contrast to spongy bones, which are filled with a trabeculae network (Fig. 4D,E). Tubular bones were identified as endochondral ossifying bones. The inner and outer bone surfaces are covered by osteoblast‐like cells and osteoblast clusters are typically absent. Osteoblasts clusters of type II were only detected at the edges of the chondroclast zone and lateral to the cartilage of the epiphysial growth plate, at the outer surface. The outer osteoblast clusters form a ring around the cartilage and have been termed bone collar cells (Hall, 1991) (Fig. 4F; cf. endochondral ossification). The ethmoid, supraoccipital, palatine, hyomandibula, hypohyal, symplectic, basiobranchials, epibranchials, pharyngobranchials and radials of the dorsal and anal fin are also tubular bones (not shown). The quadrate, on the other hand, is a mixed bone type, composed of both tubular and spongy bone parts (Fig. 2).
Chondroid bones
Chondroid bones are mineralized cartilages that are integrated into bones. They were observed at the following bone articulation sites: quadro‐articular, ethmoid‐premaxilla, hyomandibular‐prootic and hyomandibular‐opercle. In these intramembranous bones, chondroid bony tissues make up the cartilaginous socket of the joint (Fig. 5). Besides these joints, this bone type was also detected within the scleral ossicles of the eye (see also Franz‐Odendaal et al. 2007). Histologically, two types of chondroid bone were distinguishable. In the first, the chondrocytes are surrounded by a calcified matrix, which is directly embedded and not distinguishable from the underlying normal bone tissue (Fig. 5A‐C). In contrast, in the second type, underlying the calcified chondrocytes, a collagen‐rich layer was observed, connecting the chondroid bone to the bony tissue (Fig. 5E,F). However, no osteoblasts or osteoblast‐like cells were observed in either type. Osteoblasts of type II are only present at the edges of the chondroid bones (Fig. 5D), where they are differentiating into the osteoblast‐like cells. This result suggests that the chondrocytes, rather than the osteoblast‐like cells, may be directly responsible for the mineralization of this bone type. This hypothesis requires further testing.
Figure 5.
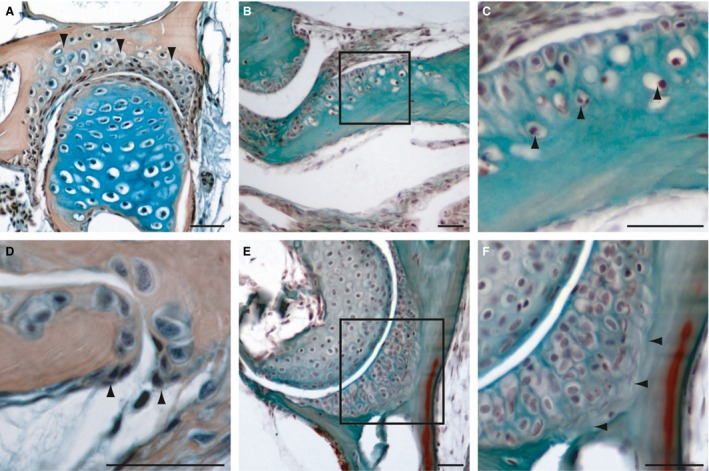
Histology of adult zebrafish chondroid bones on transversal (A, D‐F) and longitudinal sections (B,C). (A) Mallory's Trichrome and (B‐F) HBQ staining. (A) Section of the hyomandibular joint, arrowheads are indicating the chondroid bone. (B,C) Sections of the premaxillary. (C) Detail of the transition to normal bone, arrowheads are indicating bone tissue entrapped chondrocytes. (D) Detail of the hyomandibular‐opercle joint edge, arrowheads are indicating osteoblast clusters of type II. (E,F) Section of the hyomandibular‐opercle joint, detail (F) shows a collagen‐rich zone (arrowheads) between the chondroid cartilage and the bone tissue. Scale bars: 25 μm.
Discussion
Two distinct osteoblast types in zebrafish bones
The presence of osteoblast clusters at the end of growing bones in fish was previously known (Witten et al. 1997); however, up until now all clusters were presumed to be similar. Here, we identify two distinct types of osteoblast clusters in zebrafish bones, type I and type II. Moreover, we determined that type II clusters, which are smaller, are more commonly distributed over the entire fish skeleton than are type I clusters; the latter were previously identified by Witten and colleagues (1997).
Kimmel and colleagues (2010) previously hypothesized that the growth phase of bone development depends on changes in osteoblast position over development, with new osteoblast arrangements producing new patterns of matrix deposition and bone outgrowth. In support of this hypothesis, molecular studies have shown that ihha, a member of the Hedgehog family, is expressed within a small subpopulation of osteoblasts lining the antero‐ventral edge of the opercle (Huycke et al. 2012). Experimentally increasing or decreasing ihha expression led to a localized increase or decrease in bone size, respectively, through changes in pre‐osteoblast proliferation and recruitment to the bone surface (Huycke et al. 2012). Interestingly, altering ihha levels affected only the antero‐ventral region of the opercle and not the entire bone, suggesting that antero‐ventral osteoblasts may be different from other osteoblasts. In our study, we provide direct evidence for the existence of two spatially and histological distinct osteoblast types in the fish skeleton. We find that osteoblast clusters type I are present at the edge of the growing bones, where new matrix deposition and bone growth occur. These osteoblasts have the typical histological features of highly active cells. These are likely the same cells in which Huycke and colleagues (2012) observed an ihha‐ expressing subpopulation of osteoblasts. Thus, growth of teleost bones is regulated by two types of osteoblasts fine‐tuning the rate of new bone matrix deposition in a precise spatio‐temporal manner. Type II osteoblasts, on the other hand, are both more common in the skeleton and are further differentiated than type I osteoblasts.
Previous studies have shown that osteoblasts in the zebrafish have different morphologies depending on their secretory activity and on their position in the skeleton (Huysseune, 2000; Witten & Hall, 2002; Apschner et al. 2011). Secretory osteoblasts of acellular fish bones, for example, appear polarized, with a highly basiophilic cytoplasm, indicating intense protein production. These cells are thought to withdraw continuously from the bone surfaces and are thus never incorporated into the matrix, thus leading to acellular bones (Weiss & Watabe, 1979; Meunier, 1983; Ekanayake & Hall, 1987, 1988; Huysseune, 2000; Apschner et al. 2011). Based on our new data, we hypothesize about how osteoblasts become entrapped in the bone matrix as osteocytes leading to cellular bones in teleosts (discussed below).
Endochondral bone formation
It was assumed that the type of endochondral bone formation, which is well‐known from mammals, would be uncommon in small teleost species such as the zebrafish and that, if it occurs, the bony shaft would be filled with adipose tissue without building a trabecular network (Huysseune, 2000; Witten et al. 2001; Witten & Huysseune, 2009). In our study, however, we were able to show that two types of endochondral ossification occur in zebrafish. Type I endochondral ossification, the type similar to mammalian endochondral ossification, is present, albeit in a few bones (the ceratohyal and the radials of the pectoral girdle). Type II endochondral ossification leads to adipose‐filled tubular bones.
Two studies, done in the early 1990s, report that in some small fish species the process of cartilage resorption is absent during endochondral ossification. In these situations, a continuous cartilaginous core runs through the entire bone, joining the two epiphyses (Lubosch, 1910; Haines, 1934). However, we could not find any evidence that this process takes place in zebrafish.
Histological details of endochondral ossification of the epiphyseal plate in fish have up to now not been investigated. Here, we show that the initial developmental sequence of the cartilaginous epiphysial growth plate is similar in both types of endochondral ossification. However, in type II the zone of calcification and the zone of hypertrophic chondrocytes is replaced by a layer of chondroclasts. These chondroclasts have been shown previously to be tartrate‐resistant acid phosphatase (TRAP)‐positive and show the typical characteristics of resorbing cells (Witten & Huysseune, 2009).
At the outer surface of the epiphyseal plate, the bony collar osteoblasts are located, forming a ring around the epihyseal cartilage. They enlarge by the formation of membranous bony apolamellae at the outside of the bone (Stephan, 1900; Blanc, 1953; Bertin, 1958; Huysseune, 2000; Witten & Huysseune, 2007, 2009). In addition to the bone collar osteoblasts, osteoblasts with a chondrocyte morphology have also been described in the epiphyseal growth plate (Hammond & Schulte‐Merker, 2009). However, based on the location of osteoblasts in adult bones, we conclude that the osteoblasts with the chondrocyte morphology (Hammond & Schulte‐Merker, 2009) are only present during early embryonic and larval development.
Zebrafish possess cellular and acellular bones
Several studies indicate that in teleost fish, two bone types occur: cellular bones with entrapped osteocytes and acellular bones without osteocytes. In basal teleosts, the bones are cellular, whereas the bones of advanced teleosts can be acellular or anosteocytic (von Kölliker, 1859; Moss, 1961a; Ekanayake & Hall, 1987; Meunier, 1989; Meunier & Huysseune, 1992; Huysseune, 2000; Witten et al. 2004; Witten & Huysseune, 2009). Up to now, only the sheatfish (Silurus glanis) and the bonefish (Albula vulpes) (Tretjakoff & Chinkus, 1927; Moss, 1961b; Meunier, 1989) are known to possess both cellular and acellular bones. Here we show that both bone types can also occur simultaneously in the zebrafish skeleton. In addition, we show that even within the same skeletal element, there can be several bones in transition from cellularity to acellularity. This is similar to salmonids, in which varying numbers of osteocytes were observed in the cellular bones, with some completely acellular bones (Moss, 1965; Parenti, 1986). Therefore, fish skeletons may not be one of two types – cellular or acellular – as previously observed, but rather transitional stages of cellularity may be common. Further detailed histological assessments of more fish species are required to determine how widespread this phenomenon is and whether other advanced teleosts (such as the zebrafish) have a mixed bone type.
Apart from the presence/absence of osteocytes, we show that there are some other further differences, but also some similarities, between cellular and acellular bone types. For example, both are covered by a thin periosteal layer of osteoblast‐like cells and both have osteoblasts on the outer bone surfaces (Wendelaar et al. 1983; Witten et al. 1997). However, our detailed examination showed that osteoblasts are absent (or very rare) on the outer bone surfaces of acellular bones in the zebrafish.
Furthermore, we hypothesize that the osteoblast‐like cells on the bone surface/periosteal layer originate from osteoblast clusters type I and II. However, not much is known about these cells or their function. Avaron et al. (2006) and Weigele et al. (2015) indicated that osteoblast‐like cells are OP‐L positive and might therefore be involved in the deposition of bone matrix. However, these cells have few other characteristics of typical osteoblasts (Witten et al. 1997). Further studies are required to characterize fully the function of these osteoblast‐like cells.
Two origins for osteocytes in zebrafish
We show that in zebrafish bones, two histological distinct origins of osteocytes are present. One type seems to originate from the type I osteoblast clusters and is observed only in bones that grow rapidly during development, such as the opercular bone. We assume that these osteocytes represent typical entrapped osteoblasts, which fail to withdraw from the bone surfaces (Weiss & Watabe, 1979; Meunier, 1983; Ekanayake & Hall, 1987, 1988; Huysseune, 2000; Apschner et al. 2011) fast enough and are therefore trapped into the bone matrix as osteocytes.
The second type of osteocyte originates from the osteoblast‐like cells, and also becomes entrapped within the bony osseus. These cells have typically two cellular processes, from which we assume they build a lacunocanalicular system (Cao et al. 2011). However, connections between two osteocytes via cytoplasmic processes were not observed; this may, however, be due to the light microscopy method used here. Further studies are required to investigate whether this is a true lacunocanalicular system or whether these cells have other types of cellular processes connecting them to the osteoblast‐like cells on the bone surface, without connections to the adjacent embedded osteocyte. For a review of the osteocyte–osteoblast cellular connections of cellular bone see Franz‐Odendaal et al. (2006).
Bone types and their origin
It appears from our data that the occurrence of the bone types observed in this study (compact, tubular, and spongy bones) may be linked to their mode of ossification (Fig. 2, Tables 1, 2, 3). Bones developing via intramembranous ossification generally form compact bones, with only one exception detected, the angular, which is a spongy bone. In contrast to intramembranous ossification, compact, tubular and spongy bones can arise via endochondral ossification. In particular, most flat bones of the endochondral dermatocranium appear as compact bones (Table 1). The edges of a small portion of the bones beneath the cartilaginous epiphysial plates are always enclosed in adipose tissue. Furthermore, our data show that tubular and spongy bones can arise from two different modes of endochondral ossification (discussed above).
Compact bones and osteons
In this study, we provide evidence for the occurrence of acellular osteons in zebrafish, similar to those found in the acellular billfishes (Shahar & Dean, 2013; Atkins et al. 2014). The bones of teleost fish develop first as woven bone and parallel‐fibered bone. Lamellar bone develops in more mature individuals. Lamellar bone can also form osteons with a thin deposition of circumferential lamellae in most teleost species (Amprino & Godina, 1956; Enlow & Brown, 1956; Moss, 1961a,b; Smith‐Vaniz et al. 1995; Meunier et al. 2002; Witten & Hall, 2002, 2003; Atkins et al. 2014). Osteons have been not well studied in fish (Moss, 1961b), with exception of the billfishes, and more research is needed in this area.
Tubular and spongy bones
In our study, we observed two different bone types, which originate exclusively via endochondral ossification: tubular and spongy bones. We show that spongy bones develop via endochondral ossification type I (with the only exception of the anguloarticular, which is a spongy dermal bone) and tubular bones originate via type II. We also show that tubular bones lack both a bony trabecular network and other tissues (e.g. blood vessels, nerves) and are instead filled with adipose tissue (Huysseune, 2000; Witten et al. 2001; Apschner et al. 2011). This is in contrast to spongy bones, which contain trabeculae, blood vessels and nerves. Based on its histology and occurrence, we hypothesize that tubular bones are a special adaption to absorb bending forces (e.g. in the hypurals during swimming).
Chondroid bones
In the zebrafish, we detected chondroid bones almost exclusively at the articular surfaces of intramembranous bones. Chondroid bone exhibits characteristics of bone and of cartilage; it develops from osteogenic percursors and contains chondrocyte‐like cells surrounded by a bone‐like mineralized matrix (Bcresford, 1981; Huysseune, 1986; Huysseune & Verraes, 1986, 1990; Huysseune & Sire, 1990; Witten & Hall, 2002; Witten & Huysseune, 2009; Apschner et al. 2011). Chondroid bone in teleosts is associated with sites that experience mechanical stress, such as beneath articular surfaces (Huysseune & Sire, 1990; Benjamin et al. 1992; Estêvão et al. 2011). This tissue is considered to meet the support, resistance and movement demands to which articular bones are subjected (Moss, 1963; Huysseune & Sire, 1990; Benjamin et al. 1992). Another property of chondroid bone is that it has the ability to grow rapidly (Huysseune & Verraes, 1986; Gillis et al. 2005). This ability is especially important in the growth of the jaw in male Atlantic salmon (Salmo salar) during their spawning run (Witten & Hall, 2003; Gillis et al. 2005). However, based on the exclusive occurrence of chondroid bones at the articular surfaces of intramembranous bones, its function in the joints in the zebrafish skeleton is more likely to be for the attachment of cartilages in intramembranous bones than for support, resistance and movement.
Acknowledgements
We thank Brian Hall (Dalhousie University, Canada) for reviewing an earlier version of this manuscript and Beverly Hymes from Mount Saint Vincent University (Canada) for the preparation of the histological sections. We also thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and Mount Saint Vincent University for funding this research. Grant number: DG 328376.
References
- Akiva A, Malkinson G, Masic A, et al. (2015) On the pathway of mineral deposition in larval zebrafish caudal fin bone. Bone 75, 192–200. [DOI] [PubMed] [Google Scholar]
- Amprino R, Godina G (1956) Osservazioni sul nunovamento strutturale dell ‘0880 in pesci teleostei. Pub Staz Zool Napoli 28, 62–71. [Google Scholar]
- Apschner A, Schulte‐Merker S, Witten PE (2011) Not all bones are created equal – using zebrafish and other teleost species in osteogenesis research. Methods Cell Biol 105, 239–255. [DOI] [PubMed] [Google Scholar]
- Atkins A, Dean MN, Habegger ML, et al. (2014) Remodeling in bone without osteocytes: billfish challenge bone structure–function paradigms. Proc Natl Acad Sci U S A 111, 16047–16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avaron F, Hoffman L, Guay D, et al. (2006) Characterization of two new zebrafish members of the hedgehog family: atypical expression of a zebrafish indian hedgehog gene in skeletal elements of both endochondral and dermal origins. Dev Dyn 235, 478–489. [DOI] [PubMed] [Google Scholar]
- Beresford WA (1981) Chondroid Bone, Secondary Cartilage, and Metaplasia. Vienna:Urban & Schwarzenberg. Am J Surg Pathol 5 , 405. [Google Scholar]
- Benjamin M, Ralphs JR, Eberewariye OS (1992) Cartilage and related tissues in the trunk and fins of teleosts. J Anat 181, 113–118. [PMC free article] [PubMed] [Google Scholar]
- Bertin L (1958) Squelette axial In: Traité de Zoologie Vol.XIII (ed Grassé PP.), pp. 688–709. Paris: Masson et Cie. [Google Scholar]
- Blanc M (1953) Contribution a l'etude de l'osteogenese chez les poissons teleosteens. Mem Mus Natl Hist Nat 7A, 1–146. [Google Scholar]
- Cao L, Moriishi T, Miyazaki T, et al. (2011) Comparative morphology of the osteocyte lacunocanalicular system in various vertebrates. J Bone Miner Metab 29, 662–670. [DOI] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK (1987) The development of acellularity of the vertebral bone of the Japanese medaka, Oryzias latipes (Teleostei; Cyprinidontidae). J Morphol 193, 253–261. [DOI] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK (1988) Ultrastructure of the osteogenesis of acellular vertebral bone in the Japanese medaka, Oryzias latipes (Teleostei, Cyprinidontidae). Am J Anat 182, 241–249. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Brown SO (1956) A comparative histological study of fossil and recent bone tissues. Part I. Tex J Sci 8, 405–443. [Google Scholar]
- Estêvão MD, Silva N, Redruello B, et al. (2011) Cellular morphology and markers of cartilage and bone in the marine teleost Sparus auratus . Cell Tissue Res 343, 619–635. [DOI] [PubMed] [Google Scholar]
- Flint MH, Lyons MF (1975) The effect of heating and denaturation on the staining of collagen by Masson's trichrome procedure. Histochem J 7, 547–555. [Google Scholar]
- Franz‐Odendaal TA (2011) Induction and patterning of intramembranous bone. Front Biosci (Landmark Ed) 16, 2734–2746. [DOI] [PubMed] [Google Scholar]
- Franz‐Odendaal TA, Hall BK, Witten PE (2006) Buried alive: how osteoblasts become osteocytes. Dev Dyn 235, 176–190. [DOI] [PubMed] [Google Scholar]
- Franz‐Odendaal TA, Ryan K, Hall BK (2007) Developmental and morphological variation in the teleost craniofacial skeleton reveals an unusual mode of ossification. J Exp Zool B Mol Dev Evol 308, 709–721. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Witten PE, Hall BK (2006) Chondroid bone and secondary cartilage contribute to apical dentary growth in juvenile Atlantic salmon. J Fish Biol 68, 1133‐1143. [Google Scholar]
- Haines RW (1934) Epiphysial growth in the branchial skeleton of fishes. Q J Microsc Sci 2, 77–97. [Google Scholar]
- Hall BK (1986) The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morph 93, l33–l52. [PubMed] [Google Scholar]
- Hall BK (1991) Bone: embryonic development In: Encyclopedia of Human Biology (ed. Dulbecco R.), pp. 781–791, Orlando: Academic Press. [Google Scholar]
- Hall BK (2015)Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Elsevier Science Publishing Co; Pp 920. [Google Scholar]
- Hammond CL, Schulte‐Merker S (2009) Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development 136, 3991–4000. [DOI] [PubMed] [Google Scholar]
- Huycke TR, Eames FB, Kimmel CB (2012) Hedgehog‐dependent proliferation drives modular growth during morphogenesis of a dermal bone. Development 139, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysseune A (1986) Late skeletal development at the articulation between upper pharyngeal jaws and neurocranial base in the fish, Astatotilapia elegans, with the participation of a chondroid form of bone. Am J Anat 177, 119–137. [DOI] [PubMed] [Google Scholar]
- Huysseune A (2000) Skeletal system In: The Laboratory Fish (ed. Ostrander G.), pp. 307–317. London: Academic Press. [Google Scholar]
- Huysseune A, Sire JY (1990) Ultrastructural observations on chondroid bone in the teleost fish Hemichromis bimaculatus . Tissue Cell 22, 371–383. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Verraes W (1986) Chondroid bone on the upper pharyngeal jaws and neurocranial base in the adult fish Astatotilapia elegans . Am J Anat 177, 527–535. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Verraes W (1990) Carbohydrate histochemistry of mature chondroid bone in Astatotilapia elegans (Teleostei: Cichlidae) with a comparison to acellular bone and cartilage . Ann Sci Nat Zool Biol Anim 11,29–43. [Google Scholar]
- Kague E, Gallagher M, Burke S, et al. (2012) Skeletogenic fate of zebrafish cranial and trunk neural crest. PLoS ONE 7, e47394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, DeLaurier A, Ullmann B, et al. (2010) Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS ONE 5, e9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kölliker A (1859) On the different types in the microstructure of the skeletons of osseous fish. Proc R Soc London 9, 656–668. [Google Scholar]
- Laue K, Pogoda HM, Daniel PB, et al. (2011) Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am J Hum Genet 89, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubosch W (1910) Bau und Entstehung der Wirbeltiergelenke: eine Morphologische und histogenetische Untersuchung. Frankfurt: Fischer. [Google Scholar]
- Meunier FJ (1983) Les tissus osseux des Ostéichthyens. Structure, genèse, croissance et évolution. Arch doc Inst ethnol micro‐édition mus nat hist Nat, SN, 82‐600‐328, p. 200.
- Meunier FJ (1989) The acellularization process in osteichthyan bone In: Trends in Vertebrate Morphology (eds Splechtna H, Hilgers H.), pp. 443–446. Stuttgart: Gustav Fischer Verlag. [Google Scholar]
- Meunier FJ, Huysseune A (1992) The concept of bone tissue in Osteichthyes. Neth J Zool 42, 445–458. [Google Scholar]
- Meunier FJ, Journiac N, Lavoué S, et al. (2002) Caractéristiques histologiques des marques de croissance squelettique chez l'atipa Hoplosternum littorale (Hancock, 1828) (Teleostei, Siluriformes), dans le marais de kaw (Guyane Française). Bull Franç Pêche Piscicult 364, 71–86. [Google Scholar]
- Moss ML (1961a) Osteogenesis of acellular teleost fish bone. Am J Anat 108, 99–110. [Google Scholar]
- Moss ML (1961b) Studies of the acellular bone of teleost fish. I. Morphological and systematic variations. Acta Anat 46, 343–462. [PubMed] [Google Scholar]
- Moss ML (1963) The biology of acellular teleost bone. Ann N Y Acad Sci 109, 337–350. [DOI] [PubMed] [Google Scholar]
- Moss ML (1965) Studies of the acellular bone of teleost fish. V. Histology and general mineral homeostasis of fresh‐water species. Acta Anat 60, 262–276. [PubMed] [Google Scholar]
- Murciano C, Fernández TD, Durán I, et al. (2002) Ray‐interray interactions during fin regeneration of Danio rerio . Dev Biol 252, 214–224. [DOI] [PubMed] [Google Scholar]
- Parenti LR (1986) The phylogenetic significance of bone types in euteleost fishes. Zool J Linn Soc 87, 37–51. [Google Scholar]
- Shahar R, Dean MN (2013) The enigmas of bone without osteocytes. BoneKEy Rep 2, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Vaniz WF, Kaufman LS, Glowacki J (1995) Species‐specific patterns of hyperostosis in marine teleost fishes. Mar Biol 121, 573–580. [Google Scholar]
- Stephan P (1900) Recherches histologiques du tissu osseux Poissons. Bull Sci Franc Belg 33, 280–429. [Google Scholar]
- Tretjakoff D, Chinkus F (1927) Das Knochengewebe der Fische. Z Anat Entw Gesch, 83363–83396.
- Weigele J, Franz‐Odendaal TA, Hilbig R (2015) Expression of SPARC and the osteopontin‐like protein OP‐L during skeletal development in the cichlid fish Oreochromis mossambicus . Dev Dyn 244, 955–972. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Watabe N (1979) Studies on the biology of fish bone. III. Ultrastructure of osteogenesis and resorption in osteocytic (cellular) and anosteocytic (acellular) bones. Calcif Tissue Int 28, 43–56. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga SE, Lammers PI, van der Meij JC. (1983) Effects of 1,25‐ and 24,25‐dihydroxyvitamin D3 on bone formation in the cichlid teleost Sarotherodon mossambicus . Cell Tissue Res, 228, 117–126. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hall BK (2002) Differentiation and growth of kype skeletal tissues in anadromous male Atlantic salmon (Salmo salar). Int J Dev Biol 46, 719–730. [PubMed] [Google Scholar]
- Witten PE, Hall BK (2003) Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. J Anat 203, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten PE, Huysseune A (2007) Mechanisms of chondrogenesis and osteogenesis in fins In: Fins into Limbs: Evolution, Development, and Transformation (ed. Hall K.), pp. 79–92. Chicago: University of Chicago Press. [Google Scholar]
- Witten PE, Huysseune A (2009) A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev Camb Philos Soc 84, 315–346. [DOI] [PubMed] [Google Scholar]
- Witten PE, Villwock W (1997) Growth requires bone resorption at particular skeletal elements in a teleost fish with acellular bone (Oreochromis niloticus, Teleostei: Cichlidae). J Appl Ichthyol 13, 149–158. [Google Scholar]
- Witten PE, Bendahmane M, Abou‐Haila A (1997) Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae). Cell Tissue Res 287, 591–599. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hansen A, Hall BK (2001) Features of mono‐ and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol 250, 197–207. [DOI] [PubMed] [Google Scholar]
- Witten PE, Huysseune A, Franz‐Odendaal T, et al. (2004) Acellular teleost bone: primitive or derived, dead or alive? Palaeontol Assoc Newslett 55, 7–41. [Google Scholar]


