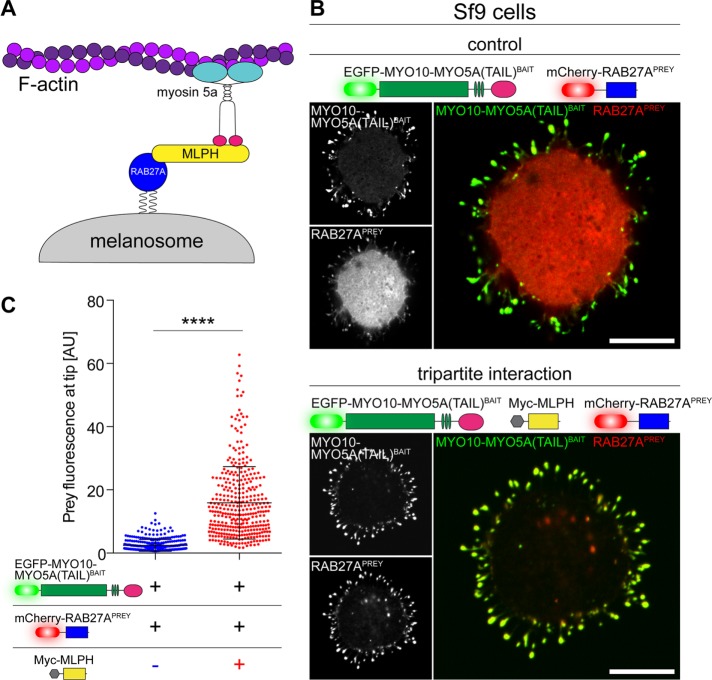
FIGURE 4:
Dissecting hierarchies in macromolecular complexes by NanoSPD. (A) Schematic of the tripartite melanosome receptor formed by MYO5A, MPLH, and RAB27A. Active RAB27A (blue) attaches to the melanosome membrane via geranylgeranyl moieties, binds its effector melanophilin (MLPH, yellow), and is recruited to the MYO5A tail domain (pink) to be trafficked along actin filaments by the motor domain (cyan). (B) NanoSPD in Sf9 cells demonstrates the requirement for MLPH in complex formation. RAB27APREY is not robustly trafficked by MYO10-MYO5A(TAIL)BAIT, despite the bait accumulating strongly at filopodial tips (top panel). Inclusion of myc-tagged MLPH (dark) results in coaccumulation of both MYO10-MYO5A(TAIL)BAIT and RAB27APREY at filopodial tips, demonstrating MLPH is required for complex formation (bottom panel). Scale bars: 10 µm. (C) ICA of RAB27APREY fluorescence at filopodial tips from B. RAB27APREY tip fluorescence is significantly increased upon coexpression of Myc-MLPH (Mann-Whitney U-test), confirming the interaction (data points are individual filopodia from three independent determinations). Data are mean ± SD. ****, p < 0.0001.