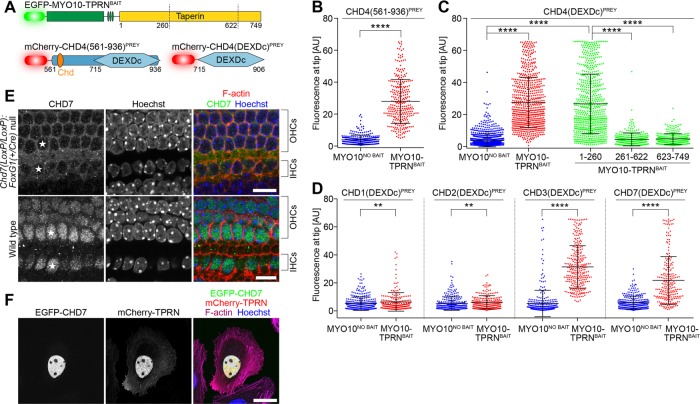
FIGURE 6:
TPRN interacts with chromodomain (CHD) family proteins in mechanosensory hair cells. (A) NanoSPD molecules to test the interaction between TPRN and CHD4, containing a DEAD-like helicase domain (DEXDc) and chromodomain (Chd). Truncation points for domain-mapping experiments are shown. (B) ICA in Sf9 cells shows that CHD4(561-936)PREY interacts at filopodial tips when expressed with MYO10-TPRNBAIT (Mann-Whitney U-test). Data are mean ± SD. (C) Domain mapping of the CHD4-TPRN interaction by NanoSPD. ICA in Sf9 cells shows the truncated CHD4(DEXDc)PREY containing the DEXDc domain (aa 715–906) by itself bound TPRN. Reciprocal truncations demonstrate that the N-terminal 260 amino acids of TPRN were sufficient to bind and traffic CHD4(DEXDc)PREY. Other truncated regions of TPRN (aa 261–622 + aa 623–749) did not bind CHD4(DEXDc)PREY (ANOVA with Tukey’s post hoc test). Data are mean ± SD from three independent determinations. (D) ICA in Sf9 cells shows binding of TPRN to the DEXDc domains of CHD3 and CHD7 but not to CHD1 or CHD2 (Mann-Whitney U-test). Data are mean ± SD, from three independent determinations. (E) Antibody labeling of CHD7 in P1 cochleae from wild-type and conditionally null Chd7 mutant mice. CHD7 labeling was absent from hair cell nuclei of conditionally null mice (white stars). OHCs, outer hair cell; IHCs, inner hair cells. Scale bars: 10 µm. (F) mCherry-TPRN (red) and EGFP-CHD7 (green) colocalize to the nucleus when overexpressed in HeLa cells. Actin filaments are labeled with Alexa Fluor 633 phalloidin (magenta), and the nucleus is labeled with Hoechst (blue). Scale bar: 25 µm. **, p < 0.01; ****, p < 0.0001.