FIGURE 1:
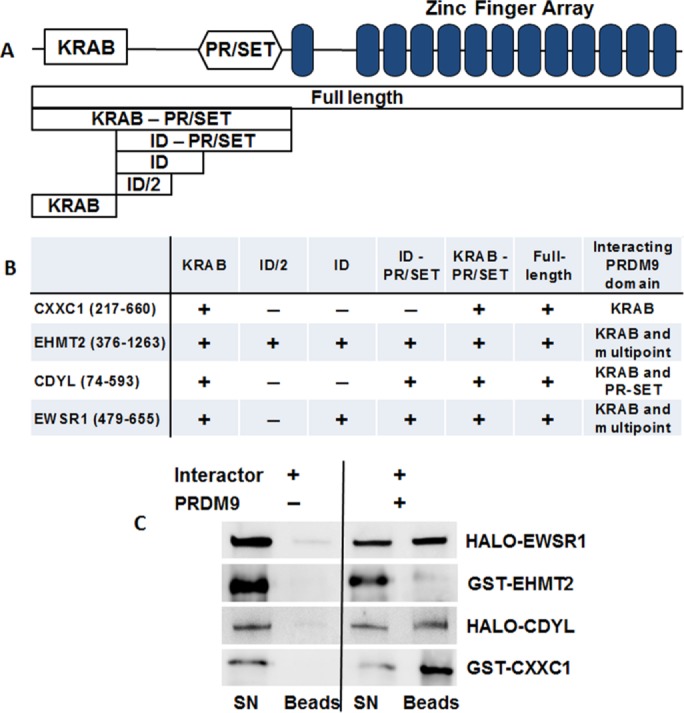
CXXC1, EWSR1, EHMT2, and CDYL directly interact with PRDM9. (A) Scheme of the domain structure of PRDM9 (top), showing the positions of the KRAB, PR/SET, and the zinc finger domains. Below are schematic representations of the full-length and deletion constructs used in the Y2H assay to map the contact points of PRDM9 interacting with the other proteins. (B) Results of the Y2H assay showing that KRAB is the major protein contact domain of PRDM9. The extents of the cloned and expressed fragments of the interactor proteins are in brackets. (C) Purified PRDM9 and its interactors bind to each other in vitro. Purified HaloTag EWSR1 and CDYL or GST-tagged EHMT2 and CXXC1 were immobilized on amylose beads alone (left) or mixed with purified full-length MBP-tagged PRDM9 (right). Specific interactions are detected by the immobilization of interactor protein on amylose beads only in the presence of MBP-tagged PRDM9. All four proteins specifically bind to PRDM9. SN, supernatant; B, beads.
