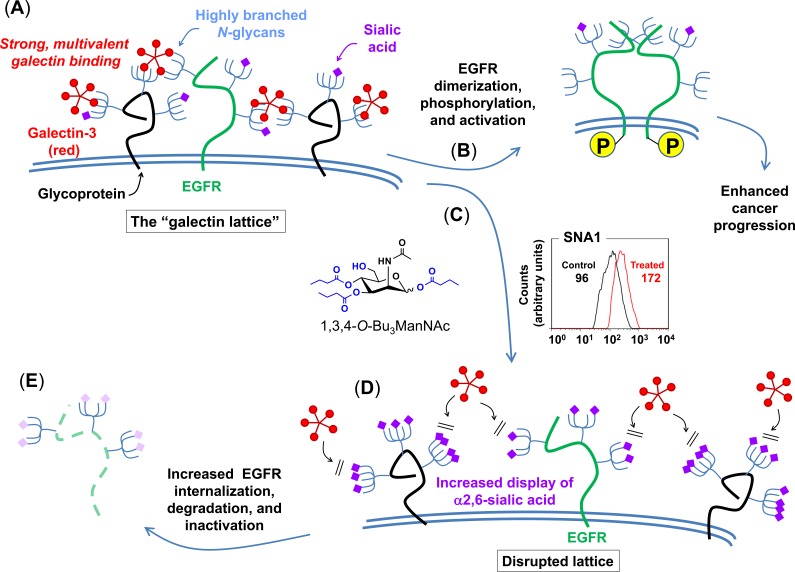
Figure 4. Proposed galectin lattice-mediated mechanism for modulation of EGFR signaling through 1,3,4-O-Bu3ManNAc treatment.
The combined modeling and early experimental results were consistent with the depicted biochemical mechanism where destabilization of the galectin lattice occurs due to the masking of galectin-binding epitopes by analog-driven increased sialylation of N-linked glycans. (A) Cancer cells often have highly organized, less-mobile surface receptors (e.g., the green structure represents EGFR while the black structures represent any other cell surface glycoprotein) in part because of a highly formed galectin lattice. (B) One result of a strong galectin lattice is lengthened residence times for EGFR on the cell surface [45], resulting in enhanced phosphorylation that lead to increased downstream EGFR signaling, which contributes to cancer progression. (C) 1,3,4-O-Bu3ManNAc treatment leads to an increase in sialylation of N-linked glycans bound to EGFR, based on lectin-staining data (specifically SNA binding as shown in the representative FACS plot depicts globally increased expression of α2,6-linked sialic upon 1,3,4-O-Bu3ManNAc treatment, which is consistent with both the data shown in Figure 1 and our previous results [4]); glycans terminated with α2,6-linked sialic acids mask galactose residues and negatively regulate galectin binding [48]. (D) In turn, reduced galectin binding decreases lattice strength, thereby increasing the surface mobility of EGFR and enhancing its removal from the cell surface [45]. (E) Ultimately – over time periods longer (e.g., 30 to 90 min) than the 2 min time frame investigated in our previous work (1) – this increased rate of internalization predicts faster inactivation of EGFR, which is computationally and experimentally demonstrated subsequently in this report.