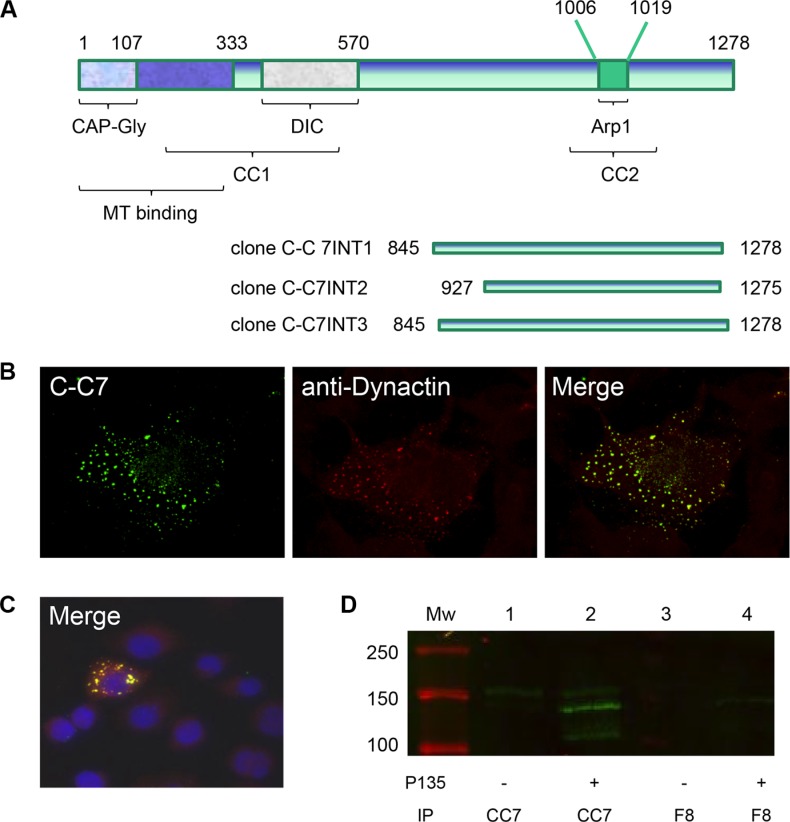
Figure 7. Dynactin-1-p150Glued is identified as a C-C7 binding partner by a yeast-2-hybrid screen.
Structural domains of dynactin-1-p150Glued and p150 domains identified by yeast-2-hybrid screens as C-C7 interactants (A). The aminoterminal domain contains a CAP-Gly domain and a coiled-coil domain which are responsible for microtubule (MT) binding and dynein binding (DIC = dynein intermediate chain). A second coiled-coil domain (CC2), encompassing a binding site for Arp1, is present in the carboxyterminal part of the protein and mediates binding to membrane components. Confocal microscopy showed colocalization of C-C7 (green) and commercial anti-dynactin-1-p150Glued (red) in COS-1 cells, transfected with the recombinant carboxyterminal domain of dynactin-1-p150Glued (B). Non-transfected cells did not stain with both antibodies (C), see negative DAPI-stained cells surrounding one transfected cell in this panel). C-C7 immunoprecipitates from extracts from CHO-s cells transfected with recombinant p135Glued were analyzed on western blot using commercial anti-dynactin-1-p150Glued as indicated (D). Note that p135 is readily precipitated by C-C7 (lane 2) but not by an irrelevant nanobody F8 (lane 4).