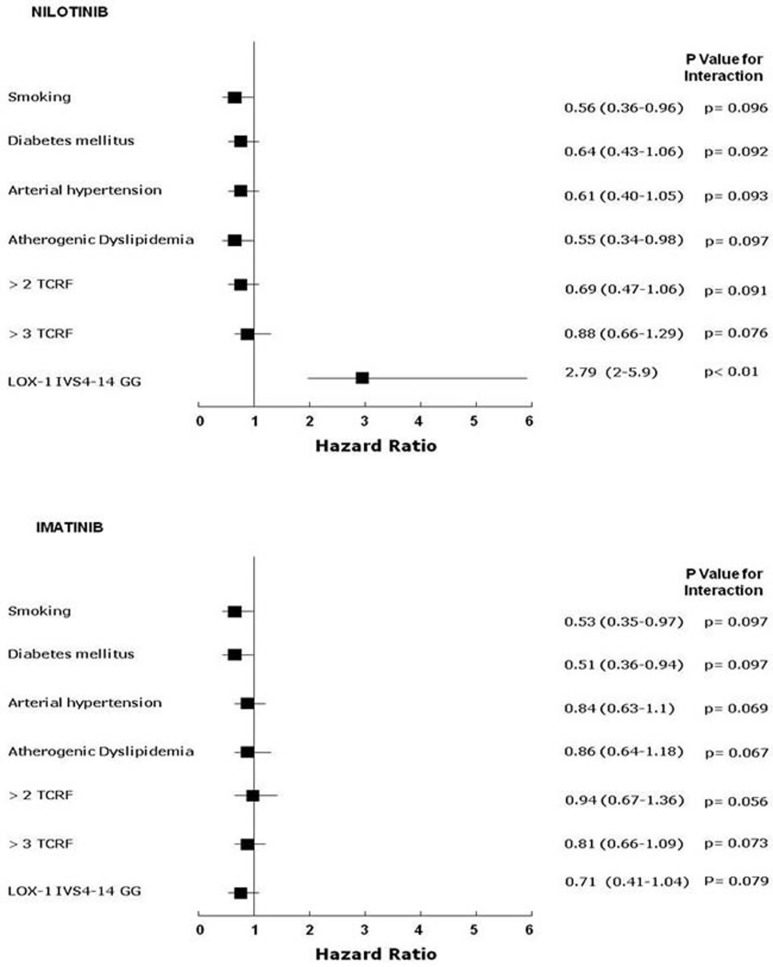
Figure 2. Relations among end-points and nonparametric data in imatinib treated (n=58) and nilotinib treated (n=52) CML patients.
Cox proportional-hazards modeling and formal test for interaction: starting from logistic regression analysis in which Y was the analyzed variable; the variable X1 and X2 were the presence or not (1 or 0) of the LOX-1 polymorphism or other non-parametric variables (history of cardiovascular classical risk factors, here named TCRF, and X3 the combination). The simplified formula for calculation was: Y = β0+β1X1+β2X2+β3X3 and the null hypothesis was tested as H0: β3 = 0. Final validation of data was assessed by a resampling technique (exact tests) and discrimination analysis by the Hosmer–Lemeshow method [G2HL= Σ10J=1 (Oj – Ej)2/Ej (1-Ej/nj) ∼X28], where nj = number of observations in the jth group, Oj = Σ1yij = observed number of positive cases in the jth group, Ej = Σpij = Expected number of positive cases in the jth group. (Each reported p is that obtained by this technique and significant if < 0.05)