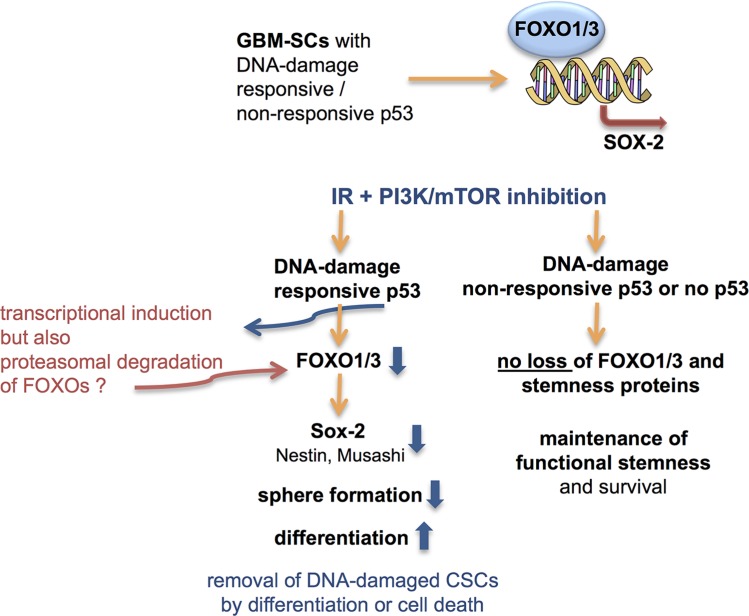
Figure 7. Proposed role of FoxOs and p53 in the control of stemness and post-treatment survival in GBM-SCs.
FoxO1/3a single and double knockdowns, pharmacological inhibition of transcriptional activity, and ChIP assays suggest that both FoxO1 and FoxO3a increase the transcription of Sox2 in GBM-SCs. In p53-proficient GBM-SCs, γIR + dual PI3K/mTOR inhibitor treatment reduces stem and progenitor marker expression after a few days while increasing differentiation marker expression, which is associated with impaired self-renewal (sphere formation) and slightly increased cell death. In p53-deficient GBM-SCs, neither FoxO proteins nor stem and progenitor markers or sphere formation decrease upon the combination treatment, and the cells survive. The loss of Sox2 expression in combination-treated, p53-proficient GBM-SCs may be caused by the loss of FoxO proteins, which may be proteolytically degraded by proteasomes upon the p53-mediated induction of Mdm2. Whether Nestin and Musashi are also directly transcriptionally regulated by FoxOs is currently unclear. However, at least the observed Nestin expression changes could also be explained by the known transcriptional regulation of Nestin by Sox2 [61, 62].