Figure 7. Anti-leukemic efficacy of dual pathway inhibition in AML.
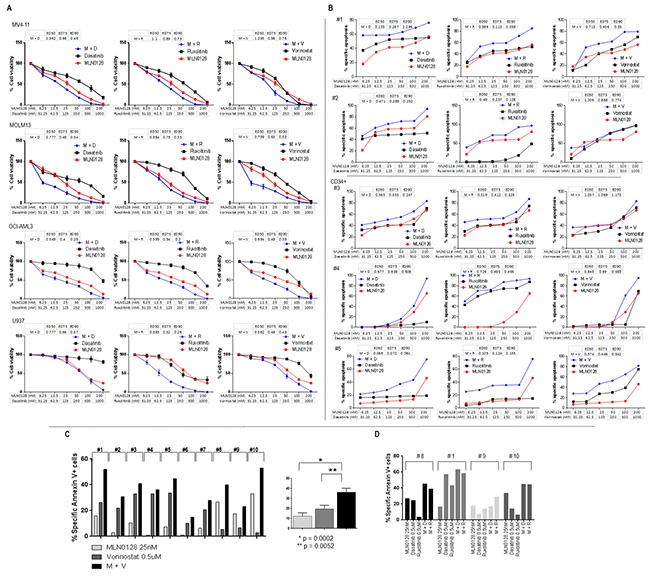
A. AML cell lines were treated with single agent and their combinations at the indicated concentrations for 72 hours. Growth inhibition in cell lines was measured by Cell Titer-Glo® luminescent cell viability assay. Drug efficacy and combination index was calculated using Calcusyn software. B. Primary AML samples were treated with single agent and their combinations at the indicated concentrations for 72 hours. Treatment-induced apoptosis in primary samples was determined by flow cytometry of annexin V/DAPI positivity in bulk (#1 and #2) or progenitor cells (CD34+) (#3, #4 and #5). Drug efficacy and combination index was calculated using Calcusyn software. C and D. Ten primary samples were treated with MLN0128 (25 nM), vorinostat (500 nM) alone or with the combination of vorinostat plus MLN0128 (C); four of the ten samples (# 8, # 1, #9, # 10) were also treated with dasatinib (500 nM), ruxolitinib (500 nM) alone or the combination of MLN0128 plus dasatinib or ruxolitinib (D). Treatment-induced apoptosis at 72 hours was detected by flow cytometry. Specific apoptosis was calculated as described in the Materials and Methods. M: MLN0128; D: dasatinib; R: ruxolitinib; V: vorinostat. Clinical information on primary AML samples shown in 7B and 7C is included in the Supplementary Table S1 combination apoptosis set B and A, respectively.
