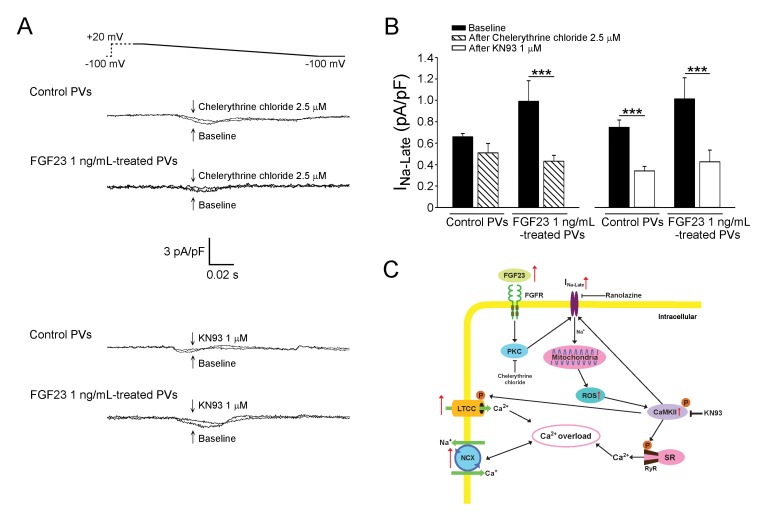
Figure 7. Signaling pathway and proposed mechanism of FGF23 on the modulation of INa-Late leading to calcium handling abnormalities.
A. Tracings and B. average data of the INa-Late from control (n = 8) and FGF23 (1 ng/mL)-treated PV cardiomyocytes (n = 8) before and after chelerythrine chloride (2.5 μM) or KN93 (1 μM) administration. *** p < 0.005. C. The INa-Late was activated by enhanced FGF23 through the signaling of PKC or the phosphorylation of CaMKII, which can be stimulated by increased mitochondrial ROS. FGF23-related PV arrhythmogenesis can be induced by calcium overload, which is produced by increased L-type calcium current (LTCC), NCX, and phosphorylation of CaMKII resulting in RyR dysfunction in the SR.