Figure 2. Crystal structure of EGFR T790M+ibrutinib and the comparison with other EGFR T790M+inhibitor co-crystal structures.
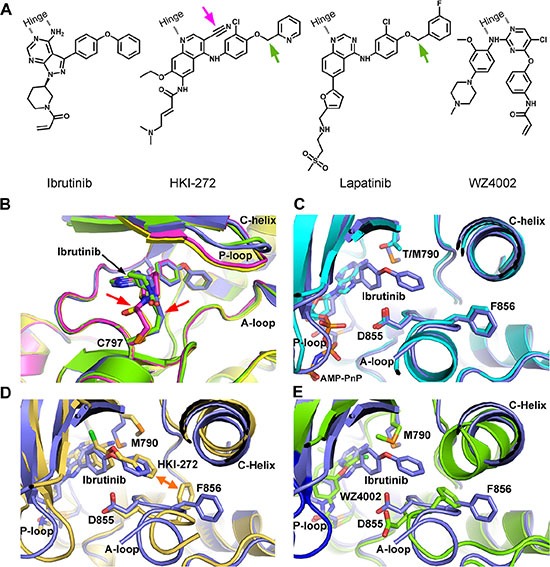
(A) Chemical structures of Ibrutinib, HKI-272, Lapatinib and WZ4002 shown in the orientation roughly indicating their binding mode to EGFR. “Hinge” indicates the hinge peptide of EGFR that connects N-lobe and C-lobe of the kinase and interacts with the inhibitors through hydrogen bonds (indicated by dashed lines). The green arrows indicate the extra methylene in HKI-272 and Lapatinib. The purple arrow indicates the cyano group of HKI-272. (B) Superimposition of the four molecules in the asymmetric unit of the EGFR T790M+ibrutinib co-crystal structure. The red arrows indicate the slight difference between the two binding modes of ibrutinib. (C) Superimposition of the EGFR T790M+ibrutinib structure (slate) and the V948R+AMP-PnP structure (drawn from PDB ID 2GS7, cyan). (D) Superimposition of the EGFR T790M+ibrutinib structure (slate) and the T790M+HKI-272 structure (yellow). The double-headed orange arrow indicates the hydrophobic interaction between the inhibitor and the Phe856 side-chain. (E) Superimposition of the EGFR T790M+ibrutinib structure (slate) and the T790M+WZ4002 structure (green).
