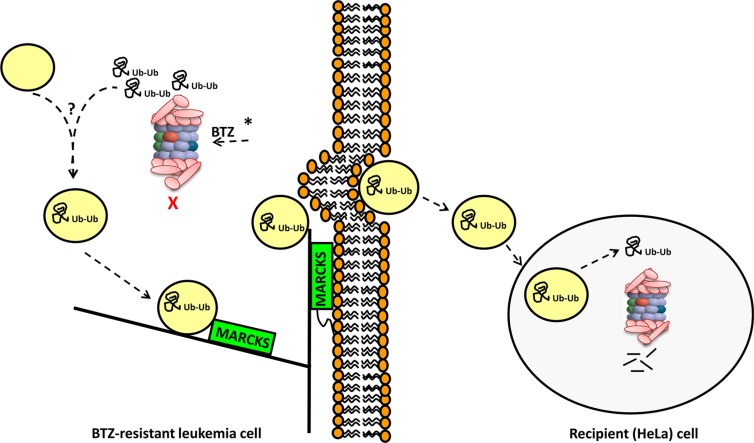
Figure 7. Summary model of the involvement of MARCKS and vesicular exocytosis of ubiquitinated proteins in BTZ-resistant leukemia cells.
CEM/BTZ cells harboring PSMB5 mutations (indicated by *) have a diminished capacity of inhibition of proteasomal catalytic activity by BTZ [29, 31]. Upon exposure of CEM/BTZ7 and CEM/BTZ200 cells to BTZ concentrations that block PSMB5 activity (30 nM and 400 nM BTZ, respectively), these cells accumulate polyubiquitinated proteins. This coincides with the biogenesis of vesicle-like structures incorporating these ubiquitinated proteins. These vesicles then traffic along actin/cytoskeleton axis to the plasma membrane with MARCKS protein serving as partner protein. Here myristoylated-anchored MARCKS facilitates exocytosis of vesicles which subsequently can be taken up by recipient (HeLa) cells. Proficient proteasomal activity in recipient cells would allow degradation of ubiquitinated proteins from BTZ-resistant cells. Through a mechanism of exocytosis-mediated extrusion of vesicles containing ubiquitinated proteins, BTZ-resistant cells can overcome proteolytic stress over a broad range of BTZ concentrations.