Abstract
Background
Chromosome 22q11.2 deletion syndrome (22q11DS) is a promising model for studying psychosis risk. Direct comparisons of psychosis features between 22q11DS and non-deleted (ND) individuals are limited by inconsistency and small samples. In the largest study to date, we compare 22q11DS to ND in comorbidities, functioning, cognition, and psychosis features across the full range of overall severity.
Methods
ND youths (n=150) aged 9-24 were matched to 22q11DS (n=150) on age and sex, stratifying for presence of psychosis-spectrum disorder. Individuals were evaluated for psychosis using the Structured Interview for Prodromal Syndromes (SIPS), and for ADHD, substance-related, and mood disorders. Differential item functioning analysis addressed whether 22q11DS differ from ND in the probability of clinically significant ratings while holding constant the overall level of psychosis.
Results
Onset of psychosis-proneness was similar among 22q11DS (mean=11.0 years) and ND (mean=12.1 years). Accounting for higher overall psychosis symptoms, 22q11DS participants were still more likely to manifest impaired stress tolerance, avolition, and ideational richness; ND were more likely to exhibit unusual thoughts, persecutory ideas, and bizarre thinking. Cognition was impaired in 22q11DS, but did not correlate with symptoms except ideational richness. Comorbid anxiety disorders were more likely in psychosis-spectrum 22q11DS; substance-related disorders were more likely in ND. GAF was similar in 22q11DS and ND, except among those with low total SIPS scores.
Conclusions
Individuals with 22q11DS share overarching similarities with ND in psychosis symptoms and age of onset for psychosis-proneness; this continues to support the 22q11DS model as a valuable window into mechanisms contributing to psychosis.
Keywords: 22q11.2 Deletion Syndrome, Psychosis, Schizophrenia, Clinical High Risk, Prodromal, DiGeorge syndrome
Introduction
Risk for psychotic illness is markedly elevated in 22q11.2 deletion syndrome (22q11DS), reaching 25% or higher by adulthood (1-4). The syndrome arises from a 1.5-3.0 megabase hemizygous deletion on the short arm of chromosome 22 in approximately 1:4000 live births, producing a variable phenotype of neuropsychiatric and physical features including cardiac, palate, endocrine, and immunologic abnormalities (5-7). Psychiatric disorders are prevalent, with increased risk for autism, attention deficit hyperactivity disorder, anxiety disorders, and psychotic disorders (1, 4, 8). The risk for psychosis represents a 25-fold increase over the general population, and 10-fold over other developmentally delayed populations (9, 10). Connections are emerging to putative genetic and cognitive mediators of risk (10-13), and 22q11DS is increasingly recognized as an informative window for understanding genetic and neurobiological substrates of psychosis risk (2, 14).
Research in psychotic illness has evolved to examine psychosis as a spectrum, with common risk factors shared across diagnoses like schizophrenia, schizoaffective disorder, and mood disorders with psychotic features (15-17). Subthreshold symptoms in the psychosis spectrum are investigated in an effort toward early identification of psychosis-proneness, with criteria defining an “at-risk mental state” or “prodrome” for individuals with significant symptomatic burden who do not meet criteria for schizophrenia spectrum disorders (17-20). Likewise, psychosis-proneness is a focus of research in 22q11DS (8, 21-25).
In earlier work comparing 22q11DS to ND, Bassett et al examined features of schizophrenia in 16 adults with 22q11DS and 46 ND adults with the illness, and found no difference in age of onset, global functioning, or prevalence and severity of hallucinations, delusions, negative and disorganized symptoms; however, individuals with 22q11DS experienced a lower prevalence of comorbid substance abuse (26). Another study of adults with schizophrenia comparing 22q11DS (n=18) and ND (n=65) also reported no difference in age of onset, positive, or negative symptoms, but found that lifetime global functioning reached lower levels in ND than in 22q11DS (27). The subthreshold psychosis-prone state was contrasted between 30 adolescents with 22q11DS and 81 ND. Assessment with the Positive and Negative Syndrome Scale demonstrated higher negative symptoms, lower general functioning, and older age of onset for the ultra-high risk state in 22q11DS (25). Another study used the Structured Interview for Prodromal Syndromes (SIPS) to compare 23 adolescents with 22q11DS to matched ND controls with and without schizotypal personality disorder (SPD); of 19 measures reflecting positive, negative, disorganized and general symptoms, 22q11DS was differentiated from SPD only by greater deficits in ideational richness and motor disturbance (28). Limited by small sample size, these few direct comparisons were conducted across broad diagnostic categories and did not account for potential differences in symptom burden. Differences in psychosis features may be either obscured or exaggerated by disparities in the overall severity of psychosis.
Here, we aim to evaluate the generalizability of psychosis-related findings in 22q11DS to ND individuals by directly comparing characteristics of psychosis symptoms between 150 youths with 22q11DS and 150 ND controls representative of their deleted counterparts. To achieve this, we enriched the non-deleted control group for psychosis symptoms through a screening process. Controls were selected to mirror the 22q11DS sample in the proportion classified as psychosis-spectrum; both consist of subjects recruited from community and medical clinic settings. We examine age of onset, psychosis symptomatology, cognition, comorbidities, and general functioning..
Methods and Materials
Sample Selection and Matching
Participants with 22q11DS and non-deleted (ND) controls aged 9-24 years were drawn from two ongoing studies, prospectively designed to facilitate direct comparisons by implementing the same phenotypic procedures. Both groups were recruited from medical clinics and community sources.. Each of the 150 participants with 22q11DS was matched to a ND control (1:1) based on age and sex; matches were made within the same psychosis category (i.e. 22q11DS classified as psychosis-spectrum were matched to ND also classified as psychosis-spectrum, and non-psychosis-spectrum 22q11DS were matched to non-psychosis-spectrum ND). The psychosis-spectrum classification includes individuals with both threshold and subthreshold levels of psychosis. Matching produced 150 ND controls with the same proportion of n=94 with psychosis symptoms and n=56 without psychosis symptoms. Race was not included as a parameter because the 22q11DS sample was predominantly Caucasian while the ND sample was largely African American. The final matched samples of 22q11DS (n=150) and ND (n=150) did not differ by age or sex (Table 1). Race and estimated socioeconomic status (SES) were significantly different between the two groups (p<0.001) – however, we were able to analyze Caucasian subsamples of the two groups with equivalent SES, generating results that do not contradict those of the whole study population, though some effects were no longer statistically significant (Supplemental Tables S3-S4, and Supplemental Figures S2-S4). Supplemental materials further detail sample selection and matching procedures.
Table 1.
Sample Measures
| Variable | 22q | ND | p | d |
|---|---|---|---|---|
| N | 150 | 150 | - | - |
| Mean Age (yrs ± SD) | 15.3 ± 4.2 | 16.0 ± 3.6 | N.S. | −0.16 |
| Psychosis Spectrum (%) | 94 (63%) | 94 (63%) | - | - |
| Psychosis-Prone | 83 (55%) | 85 (57%) | N.S. | - |
| Psychosis | 11 (7%) | 9 (6%) | N.S. | - |
| Mean Onset (yrs ± SD) | 11.0 ± 4.0 | 12.1 ± 3.2 | N.S. | −0.31 |
| SIPS Total (score ± SD) | 20.3 ± 13.9 | 14.7 ± 10.9 | <0.01 | 0.45 |
| GAF (score ± SD) | 62.8 ± 14.4 | 70.0 ± 14.5 | <0.001 | −0.50 |
| Sex | ||||
| Male (%) | 92 (61%) | 84 (56%) | N.S. | - |
| Female (%) | 58 (39%) | 66 (44%) | N.S. | - |
| Race | <0.001 | - | ||
| Caucasian (%) | 130 (87%) | 52 (35%) | <0.001 | - |
| African American (%) | 11 (7%) | 81 (54%) | <0.001 | - |
| Mixed (%) | 6 (4%) | 16 (11%) | N.S. | - |
| Asian (%) | 2 (1%) | 1 (1%) | N.S. | - |
| Other (%) | 1 (1%) | 0 (0%) | N.S. | - |
| Reading Proficiency (±SD) | 89.2 ± 13.5 | 100.5 ± 15.4 | <0.001 | −0.78 |
| Education (yrs ± SD) | ||||
| Proband | 7.9 ± 3.6 | 9.2 ± 3.4 | <0.05 | −0.38 |
| Mother | 14.8 ± 2.3 | 14.3 ± 2.4 | N.S. | −0.21 |
| Estimated Income (±SD) | 75,030 ± 31,053 | 51,848 ± 28,415 | <0.001 | 0.78 |
Note: 22q11DS=22q11.2 deletion syndrome; ND=non-deleted; SOPS=scale of prodromal symptoms; GAF=global assessment of function; SD=standard deviation; Yrs=years; d=Cohen's d for effect size; N.S.=non-significant. Mean age, mean age of onset, SOPS total, GAF, reading proficiency, and education were compared using Student's t-test. Proportions of psychosis spectrum, sex, and race were compared using Fisher's exact test. All comparisons were two-sided with significance threshold of p=0.05.
Instruments and Measures
Both groups were assessed for psychosis and other psychopathology with the complete Structured Interview for Prodromal Syndromes (SIPS; 29, 30) and parts of the Kiddie-Schedule for Affective Disorders and Psychosis (K-SADS; 31), as well as for medical, social and treatment histories. K-SADS sections assessed DSM-IV psychosis, mood, substance related disorders, and ADHD. Family history (FH) of psychopathology in first-degree relatives was assessed using an abbreviated version of the Family Interview for Genetics Studies (32).
Reading proficiency was calculated for each participant using the Wide Range Achievement Test 4 reading subtest (33). Other cognitive measures were assessed using the Penn Computerized Neurocognitive Battery (CNB), which has been extensively characterized (34-36). Cognition in 22q11DS and ND is compared with twelve neurocognitive tasks assessing four cognitive domains, including executive function, episodic memory, complex cognition, and social cognition (Supplemental Table S1). Supplemental materials further describe the above instruments and measures.
Scoring and Consensus Diagnosis
Elicited clinical symptoms were rated according to the 19 items on the Scale of Prodromal Symptoms (SOPS) with standardized anchors on a 7-point scale: 0=absent, 1=questionably present, 2=mild, 3=moderate, 4=moderately severe, 5=severe (but not psychotic), 6=severe and psychotic/extreme (29, 30). Only symptoms occurring in the last six months were considered. Threshold psychotic disorders were determined using DSM-IV-TR criteria (37). We additionally established criteria for “psychosis-proneness” to include individuals with one or more clinically significant positive subthreshold symptoms (with or without recent onset or worsening), as well as those with 2 or more significant negative and disorganized symptoms. All individuals who were psychosis-prone or psychotic were considered a part of the “psychosis-spectrum.”
Criteria were tabulated for diagnoses of ADHD, mood disorders, and substance related disorders based on K-SADS. Diagnoses were assigned based on DSM-IV-TR criteria (37). Narrative case summaries were composed for each subject and presented at consensus case conferences where SOPS scores and diagnoses were finalized. Global assessment of function (GAF) was also determined by consensus based on overall psychological, social, and occupational functioning according to SIPS anchors (29, 30). Additional descriptions of scoring and diagnostic procedures can be found in the supplement.
Eleven 22q11DS and nine ND participants met criteria for psychotic disorders including schizophrenia (22q11DS n=5; ND n=1), psychosis NOS (22q11DS n=3; ND n=4), delusional disorder (22q11DS n=2; ND n=1), and major depressive disorder with psychotic features (22q11DS n=1; ND n=3). Age of onset for psychosis-proneness and psychosis were determined retrospectively during the interview, and assigned via consensus review. Age of onset for psychosis-proneness was available for n=66 22q11DS and n=68 ND; age of onset for psychosis was available for n=4 22q11DS and n=8 ND. No value was recorded for the approximately one third of individuals for whom age of onset was uncertain for either psychosis-proneness or psychosis.
Data Analysis and Differential Item Functioning
Comparisons of continuous clinical and demographic variables were conducted using Student's t-tests, and effect sizes were calculated using the standard formula for Cohen's d while categorical variables were analyzed using Fisher's exact test. CNB accuracy scores by domain were normalized against ND subjects without psychosis-spectrum. An overall cognition score was calculated from the normalized mean of all four domains. Differences in mean cognitive domain scores were calculated via pairwise Student's t-tests among the following: 22q11DS with psychosis-spectrum, 22q11DS without psychosis-spectrum, ND with psychosis-spectrum and ND without psychosis-spectrum. Relationships between cognition and SOPS items were evaluated using separate multiple linear regressions of the ordinal SOPS item score on each neurocognitive domain, while covarying for group (22q11DS vs. ND). P-values were adjusted for multiple comparisons and significance threshold was two-tailed with the probability of a Type I error set to 5%. To compare GAF between 22q11DS and ND along the full range of severity of psychosis-spectrum symptoms, GAF was linearly regressed on total SOPS score, group, and group × total SOPS score (38).
Differential item functioning (DIF) occurs when two groups have different probabilities of endorsing a certain response on that item even after holding constant the overall construct of interest (39). Here, we apply DIF to address the following: when holding constant the overall burden of psychosis-spectrum symptoms (as reflected by the total number of clinically significant SOPS items), do individuals with 22q11DS differ from ND in the probability of clinically significant ratings (≥3) on any given SOPS item? Scores were dichotomized at the level of clinical significance (0-2 vs. 3-6). Four common methods for determining DIF were employed, with threshold for significant DIF set at ≥3 of 4 methods (Supplemental Table S2; Caucasian subsamples Supplemental Table S3). Supplemental materials provide further details on our application of DIF and other statistical procedures.
Results
Comparison of Demographic and Clinical Features
Table 1 compares 22q11DS and ND on multiple demographic measures. As expected given the matching procedure, the groups were equivalent in age, sex, and proportions of psychosis spectrum. More participants with 22q11DS were Caucasian compared to ND (87% vs. 35%; p<0.001) and fewer were African American (7% vs. 54%; p<0.001). Estimated household income was higher in 22q11DS ($75,030 vs. $51,848, p<0.001). Mean reading proficiency (p<0.001; d=−0.78) and years of completed education (p<0.05; d=−0.38) were significantly lower in 22q11DS compared to ND, without a corresponding difference in maternal education level (p=0.11, d=−0.21).
Clinical measures were also compared (Table 1). The mean onset of psychosis-spectrum symptoms was 11.0 ± 4.0 years in 22q11DS, and did not differ significantly from 12.1 ± 3.2 years in ND (p=0.08, d=−0.31). Onset of psychotic illness was available for a small number of participants (n=12), and also did not differ (p=0.40; d=0.63). Means were 16.0 ± 0.8 years in 22q11DS and 14.1 ± 4.1 years in ND. Mean total SOPS score was higher in 22q11DS (p<0.01; d=0.45). Similarly, the number of clinically significant items endorsed was higher in 22q11DS (mean=4.7 ± 4.0) compared to ND (mean=2.8 ± 2.8; p<0.001; d=0.56).
Limiting these analyses to Caucasian subsamples (Supplemental Table S4), there was no longer a difference in estimated income or mean education level. All other findings were consistent: individuals with 22q11DS had similar age of onset for psychosis-spectrum symptoms, higher total SOPS score, and lower reading proficiency.
Differential Item Functioning on SOPS Items
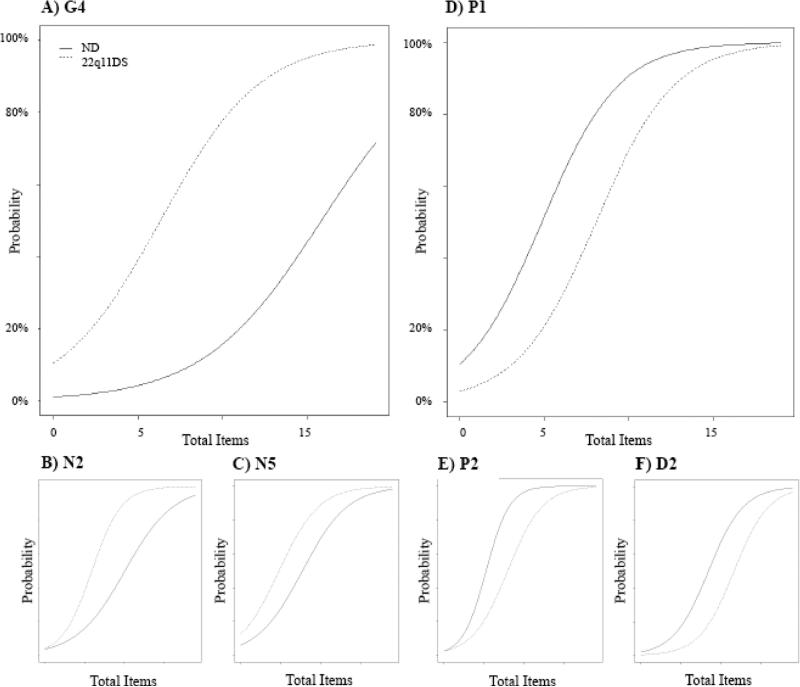
Six SOPS items measuring symptoms of psychosis were found to have significant DIF (Supplemental Table S2). On average, individuals with 22q11DS endorsed higher total SOPS scores than ND; accounting for this, 22q11DS were still more likely than ND to endorse clinically significant avolition (N2), deficit in ideational richness (N5), and impaired tolerance to normal stress (G4; Figure 1A-C). Of these, the higher likelihood of impaired stress tolerance is especially pronounced (Figure 1A). For example, an ND participant with a total of 10 clinically significant SOPS items has <20% probability of scoring ≥3 on G4 while a 22q11DS participant also with 10 clinically significant SOPS items has ~80% probability of significant impairment in stress tolerance. At no point do the curves overlap, suggesting 22q11DS are more likely than ND to experience impaired stress tolerance whether they have few psychosis symptoms or many. This is similarly true for avolition (N2, Figure 1B) and impairment in ideational richness (N5, Figure 1C).
Figure 1. SOPS Items Showing Differential Item Functioning.
Compared to ND at the same total level of symptomatology, individuals with 22q11 are more likely to have clinically significant impairment in stress tolerance (G4), avolition (N2), and ideational richness (N5) while they are less likely to have unusual thought content (P1), persecutory ideas (P2), and bizarre thinking (D2). Y-axis illustrates probability of clinically significant symptoms for that item. X-axis represents the number of total clinically significant items endorsed by a participant. 22q11DS=22q11.2 deletion syndrome; ND=non-deleted; SOPS=scale of prodromal symptoms
In contrast, ND were more likely than 22q11DS to have clinically significant unusual thought content and delusional ideas (P1), suspiciousness and persecutory ideas (P2), and bizarre thinking (D2; Figure 1D-F). This was also true for the full range of symptom quantity.
Significant DIF continued to exist for G4 and N5 when these analyses were limited to Caucasian subsamples (Supplemental Table S3; Supplemental Figure S2). DIF for N2, P1, P2, and D2 were no longer significant, but the pattern of differences in ratings was similar to that observed in the total sample.
Cognition and Relationship to Psychosis Symptoms
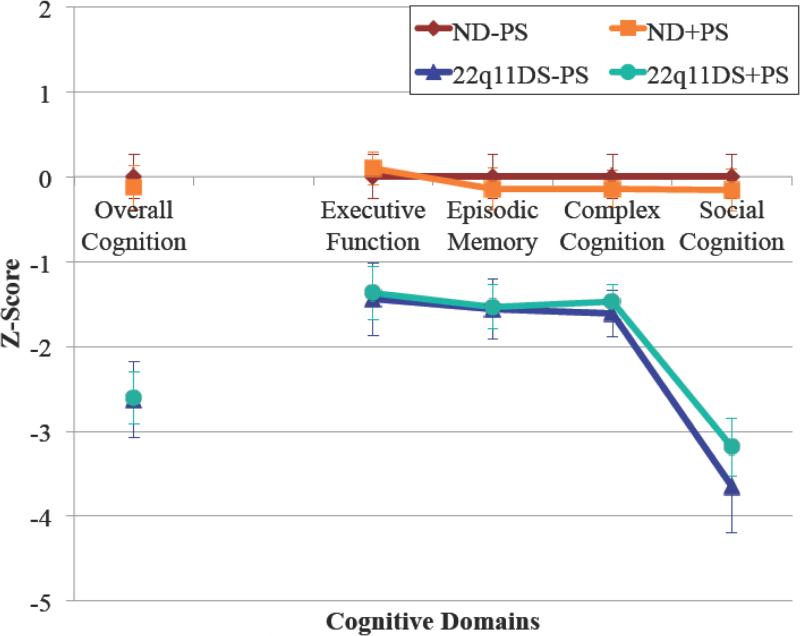
Cognition was significantly impaired for participants with 22q11DS compared to ND individuals regardless of psychosis-spectrum category (P<0.001 for all comparisons). On the other hand, there were no significant differences in cognition between those with compared to those without psychosis-spectrum among participants with either 22q11DS or ND. This was the case for accuracy scores corresponding to each of the four cognitive domains (executive function, episodic memory, complex cognition and social cognition) as well as an overall measure of cognition incorporating accuracy scores from all domains (Figure 2).
Figure 2. Cognition in 22q11.2 Deletion Syndrome and Non-Deleted Individuals.
Accuracy scores for 12 tasks assessing four cognitive domains (executive function, episodic memory, complex cognition, and social cognition) were normalized against ND individuals without psychosis-spectrum (n=56). Individuals with 22q11DS were significantly impaired in all cognitive domains as well as in the overall composite score compared to ND (p<0.001 for all comparisons). There were no differences between those with psychosis-spectrum versus those without for either 22q11DS or ND. Pairwise comparisons were made using Student's t-test with significance threshold p=0.05. Error bars represent 95% confidence intervals. 22q11DS=22q11.2 deletion syndrome; ND=non-deleted; +PS=with psychosis-spectrum; −PS=without psychosis-spectrum
After covarying for group, five of the six SOPS items showing DIF (P1, P2, N2, D2, G4) were not significantly correlated to any domain of cognition (Supplemental Table S5). N5 (ideational richness) was significantly predicted by executive function (p=0.04), complex cognition (p=0.01), and the overall composite score (p=0.002).
Comorbidities of Psychosis-Spectrum Individuals
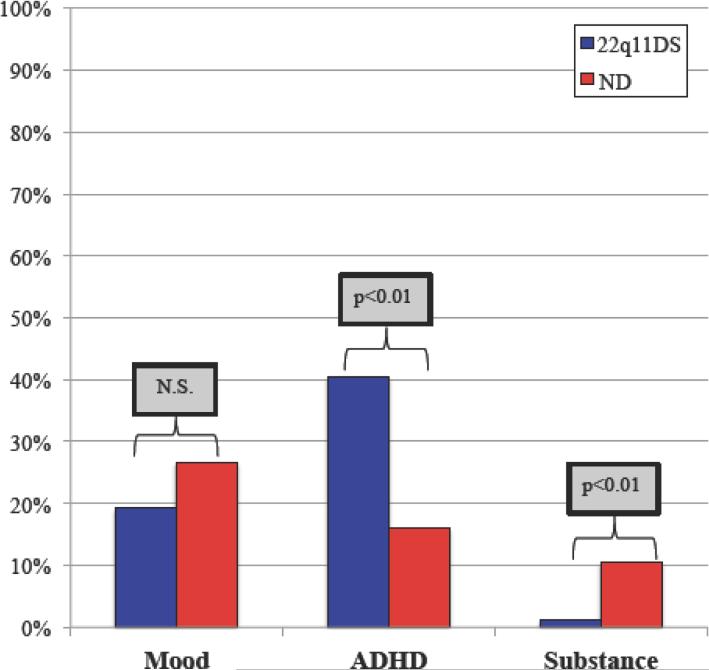
Prevalence of comorbid disorders differed for psychosis-spectrum individuals with 22q11DS compared to psychosis-spectrum ND (Figure 3). ADHD was more prevalent in 22q11DS (40%) compared to ND (16%; p<0.01) while the opposite was true for substance related disorders, which were more common in ND (11% vs. 1%; p<0.01). There was no significant difference in the prevalence of comorbid mood disorders in psychosis-spectrum 22q11DS (19%) compared to ND (27%; p=0.30).
Figure 3. Comorbidities of Psychosis-Spectrum Individuals with 22q11DS and ND.
Mood disorders occur in similar prevalence, but individuals with 22q11DS who are psychosis-spectrum are more likely to have comorbid ADHD (p<0.01) and less likely to have comorbid substance disorders (p<0.01). Prevalence of comorbidities were compared using two-sided Student's t-test with significance threshold set at p=0.05. 22q11DS=22q11.2 deletion syndrome; ND=non-deleted; N.S.=non-significant; Mood=mood disorders including major depression, bipolar disorder, dysthymia, and unspecified depressive and mood disorders; ADHD=attention deficit hyperactivity disorder; Substance=abuse or dependence on alcohol or illicits including hallucinogens, opioids, anxiolytics, and cocaine.
In Caucasian subsamples, mood disorders continued to be statistically similar while substance-related disorders continued to be more prevalent in ND. However, ADHD no longer differed between the groups (Supplemental Figure S3).
Global Assessment of Function
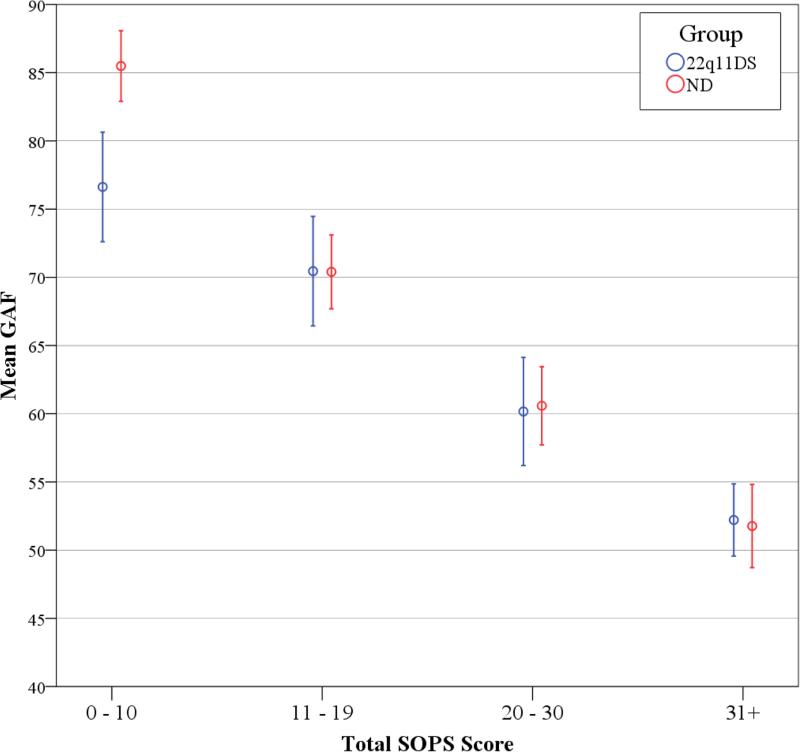
GAF scores were overall lower in 22q11DS at a mean of 62.8 compared to 70.0 in ND (Table 1: p<0.001; d=−0.50), but regression of GAF on total SOPS score showed a significant group × total SOPS score interaction (p<0.001; Figure 4). The difference in GAF between 22q11DS and ND occurs only for those with low total SOPS scores. A similar relationship existed for Caucasian subsamples (Supplemental Figure S3).
Figure 4. Global Assessment of Function and Total SOPS Score.
Mean GAF is plotted against total SOPS score for 22q11DS (blue) and ND participants (red). Total SOPS score was segmented into 4 groups, ranging from 0-10, 11-19, 20-30, and 31 and above. GAF=global assessment of function; SOPS=scale of prodromal symptoms; 22q11DS=22q11.2 deletion syndrome; ND=non-deleted.
Discussion
We directly compared clinical features, symptomatology, cognition, comorbidity, and general functioning between 150 individuals with 22q11DS and 150 age and sex-matched ND individuals. To our knowledge, this is the largest such comparison to date. Ascertained from general medical clinics and community sources, this sample is relatively representative of young people with 22q11DS. ND controls were recruited from similar sources. Assessment methodologies were prospectively standardized and discriminating statistical methods were employed. Comparison of psychosis features was maximally facilitated by selecting a ND comparison sample, enriched for the presence of psychosis, that mirrored the proportion of psychosis-spectrum individuals with 22q11DS. This design, while not suitable for comparing the prevalence of psychosis symptoms between 22q11DS and ND, allowed us to investigate noteworthy similarities and differences in the manifestation of psychosis in 22q11DS and ND.
Age of onset for psychosis-proneness was similar for 22q11DS and ND at 11.0 and 12.1 years, respectively. For threshold psychosis, there was no difference in onset among 12 subjects for whom these data were available. Similarly, two prior studies found mean age of onset for schizophrenia to be around 20 years and to be consistent between 22q11DS and ND adults (26, 27). In contrast, Armando et al (25) reported later onset for clinical high risk in 22q11DS (16.4 years; n=30) compared to ND (14.8 years; n=81). Like these studies, our measures are limited by potential inaccuracies of retrospective recall. When conversion to psychosis in 22q11DS was prospectively determined, mean age was 17.7 ± 4.2 years (10). As in 22q11DS, average age of onset for clinical-high risk in ND has been reported at around 16 years (40). Our reported mean is younger most likely due to the young age of our study sample. Additionally, we employed more inclusive critieria for defining the at-risk state by incorporating negative and disorganized symptoms. These nonspecific symptoms are postulated to precede positive symptoms in the development of psychosis (41), but they nevertheless warrant consideration as indicators of increased risk. As schizophrenia is increasingly understood as a disorder of aberrant neurodevelopment, the similar age of onset in 22q11DS compared to ND suggests the possibility that the same faulty developmental processes are at play.
Accounting for overall higher SOPS scores in 22q11DS, the two groups had similar likelihood of having clinically significant symptoms on the majority of SOPS subscales. There were exceptions. Individuals with 22q11DS were more likely to experience significant levels of stress intolerance, avolition and reduced ideational richness, while ND were more likely to experience significant unusual thought content, persecutory ideas and bizarre thinking. The difference for stress tolerance was particularly pronounced, which is of potential relevance to the finding that anxiety may be predictive of conversion to psychosis in 22q11DS (10, 42, 43). Notably, the items more commonly clinically significant in ND (holding overall psychosis constant) reflected delusional ideations. It is worth considering whether deficits in ideational richness in 22q11DS may manifest as decreased ability to report delusional thought content. Alternatively, the distress associated with psychosis may be manifested as less-defined symptoms of subjective stress. Pertinent negative findings include the thirteen items for which DIF was not found; 22q11DS do not markedly differ from ND in the likelihood of clinically significant perceptual abnormalities, disorganized speech and behavior, expression and experience of emotions, social anhedonia, occupational functioning and other symptoms. These results build on prior studies, which did not account for overall severity of symptoms. Two studies of adults with schizophrenia found no differences in symptom domains, while two studies including adolescents with subthreshold symptoms suggested that individuals with 22q11DS may experience higher negative symptoms, impaired ideational richness, and motor disturbances (25-28). Together with our results, available comparisons suggest individuals with 22q11DS experience largely comparable symptoms of psychosis to ND individuals with notable exceptions related to stress tolerance and ideational richness. This also proposes the possibility that underlying neurobiological pathways producing psychosis may be shared.
Cognition in 22q11DS was impaired compared to ND for all cognitive domains, but a significant difference between those with and those without psychosis-spectrum did not emerge. While cognitive deficits in 22q11DS are not novel, they are potentially informative; impairments in cognition have been associated with eventual conversion to psychosis (10, 11, 42). Greater deficits in multiple domains were also reported by Shapiro et al (28). While cognitive deficits are expected to be associated with psychosis-spectrum disorders, effect sizes are variable by age and smaller for individuals with milder illness in the ND population (44, 45). The absence of an effect on cognition from psychosis-proneness in our sample is likely due to the subsyndromal illnesses and their young age (which is associated with instability in the presence of psychosis symptoms). Monks et al also found no difference in IQ between individuals with 22q11DS and with versus without schizophrenia (27). Importantly, the absence of a significant difference cross-sectionally does not preclude cognitive deficits being associated with eventual conversion to psychosis. Furthermore, with the anticipated exception of ideational richness, psychosis symptoms that had shown significant DIF did not correlate with cognition independent from the presence of the deletion. This suggests that the differences between 22q11DS and ND in endorsement of delusional ideas and impaired stress tolerance are unlikely to be due to differences in cognition.
Like Bassett et al, we found that psychosis-spectrum 22q11DS were less likely than ND to meet criteria for substance-related disorders (26), whereas comorbid ADHD was more likely. Mood disorders did not differ. It is interesting to consider whether these asymmetries arise from indepedent biological and psychosocial factors or if they reflect processes related to psychosis vulnerability. For example, does 22q11DS confer a fundamental deficit in social cognition that underlies both psychosis-risk and lower comorbidity of substance-related disorders? ADHD in ND youth has been associated with subsequent increased risk for psychosis (46-48). In both populations, comorbid attention deficits may contribute to or reflect the emergence of psychosis symptoms.
Mean GAF scores were lower in 22q11DS than ND, and therefore consistent with the findings of Monks et al and Armando et al (25, 27). Bassett et al, however, found no significant difference (26). Our large sample size enabled us to investigate an interaction between GAF, group, and total SOPS score. We found that individuals with 22q11DS and few symptoms of psychosis (total SOPS score 0-10) function more poorly than ND in the same range; however, this difference disappears at moderate and higher ranges of psychosis burden. This may suggest that psychological, social, and occupational functioning are similarly affected in 22q11DS and ND by increasing burdens of psychosis symptoms. However, intellectual disability and the prevalence of other psychiatric disorders enforce a lower ceiling on functioning in 22q11DS.
All participants were assessed concurrently and cohort effects are therefore minimized. Samples of 22q11DS and ND controls were selected and matched to optimize our ability to compare psychosis features including associated symptoms like impaired tolerance to normal stress. This design necessarily prevents us from studying prevalence of psychosis or comparing overall severity in the two populations. Additionally, some sample differences should be considered. Participants with 22q11DS were predominantly Caucasian (87%), consistent with other studies of this genetic disorder. It is uncertain whether 22q11DS is truly less prevalent in other races or rather, underdiagnosed (3, 28, 49). In contrast, ND individuals (54% African American) were recruited from the greater Philadelphia area, and reflect local diversity. As expected, analyses limited to Caucasian participants were less powerful and some results were no longer significant. Education level was no longer lower for individuals with 22q11DS, and DIF was no longer present for N2, P1, P2, and D2. The prevalence of ADHD was also equivalent. However, given the overall consistency of major findings, there is limited evidence to suggest that race or SES were significant confounders. Participants with 22q11DS had also completed fewer years of schooling and scored lower in reading proficiency compared to same-aged ND controls. Though the differences were significant statistically, these measures of academic achievement were not drastically divergent; ND superceded 22q11DS by ~10% in reading proficiency and 1 year in education level. Additionally, while our utilization of 1:1 matching controlled for key known variables, there is the possibility of introducing unknown confounders.
Despite these limitations, we contribute notable insights in the emergence of psychosis in 22q11DS as it relates to the ND population. Chromosome 22q11DS is one of the highest biologic risk factors for psychosis – it is reliably diagnosed and relatively genetically homogenous compared to the overall population of individuals with psychosis, therefore potentially enhancing signal-to-noise in research aimed at isolating key contributors to psychosis risk. Longitudinal followup of youths with 22q11DS is likely to capture the emergence of psychosis, as well as its exacerbating and protective factors. Generalizability to the general population requires that psychosis features be similar between 22q11DS and ND. We present direct comparisons between 22q11DS and ND controls with substantially larger samples and finer levels of analyses than preceding studies. Psychosis in 22q11DS is distinguished from that of ND indviduals primarily by lower comorbidity with substance abuse and differences in symptoms related to stress tolerance and ideational richness, which are not accounted for by cognitive deficits. However, the majority of psychosis symptoms are endorsed with equal likelihood in 22q11DS and ND when accounting for overall symptom burden and they progress on a similar developmental timecourse. This further supports 22q11DS as an important model for studying psychosis risk.
Supplementary Material
Acknowledgements
Funding was received from NIH grants: MH087626, MH087636, MH089983, MH 089924; Doris Duke Charitable Foundation Clinical Research Fellowship (SXT); T32 MH MH019112 (JJY); and K08 MH079364 (MEC). We thank the participants and their families, Kosha Ruparel, Allison Port, Daneen A. Whinna, R. Sean Gallagher, Kelly Peters, Catherine Conroy, Amy Cassidy, and Karin Borgmann-Winter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Schneider M, Debbané M, Bassett AS, Chow EW, Fung WLA, van den Bree MB, et al. Psychiatric Disorders From Childhood to Adulthood in 22q11. 2 Deletion Syndrome: Results From the International Consortium on Brain and Behavior in 22q11. 2 Deletion Syndrome. American Journal of Psychiatry. 2014;171:627–39. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 Deletion Syndrome as a Window into Complex Neuropsychiatric Disorders Over the Lifespan. Biol Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Curr Psychiatry Rep. 2008;10:148–157. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 5.Yi JJ, Tang SX, McDonald-McGinn DM, Calkins ME, Whinna DA, Souders MC, et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;165:137–147. doi: 10.1002/ajmg.b.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry. 2014;19:1205–1211. doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, et al. The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- 8.Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan VA, Leonard H, Bourke J, Jablensky A. Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry. 2008;193:364–372. doi: 10.1192/bjp.bp.107.044461. [DOI] [PubMed] [Google Scholar]
- 10.Gothelf D, Schneider M, Green T, Debbané M, Frisch A, Glaser B, et al. Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry. 2013;52:1192–1203. e3. doi: 10.1016/j.jaac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72:377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalbrzikowski M, Lazaro MT, Gao F, Huang A, Chow C, Geschwind DH, et al. Transcriptome Profiling of Peripheral Blood in 22q11.2 Deletion Syndrome Reveals Functional Pathways Related to Psychosis and Autism Spectrum Disorder. PLoS One. 2015;10:e0132542. doi: 10.1371/journal.pone.0132542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gothelf D, Law AJ, Frisch A, Chen J, Zarchi O, Michaelovsky E, et al. Biological effects of COMT haplotypes and psychosis risk in 22q11.2 deletion syndrome. Biol Psychiatry. 2014;75:406–413. doi: 10.1016/j.biopsych.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addington AM, Rapoport JL. Annual research review: impact of advances in genetics in understanding developmental psychopathology. J Child Psychol Psychiatry. 2012;53:510–518. doi: 10.1111/j.1469-7610.2011.02478.x. [DOI] [PubMed] [Google Scholar]
- 15.Barch DM, Bustillo J, Gaebel W, Gur R, Heckers S, Malaspina D, et al. Logic and justification for dimensional assessment of symptoms and related clinical phenomena in psychosis: relevance to DSM-5. Schizophr Res. 2013;150:15–20. doi: 10.1016/j.schres.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- 17.Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salokangas RKR, Ruhrmann S, von Reventlow HG, Heinimaa M, Svirskis T, From T, et al. Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr Res. 2012;138:192–197. doi: 10.1016/j.schres.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- 21.Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, et al. Subthreshold psychotic symptoms in 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. 2014;53:991–1000. e2. doi: 10.1016/j.jaac.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2010;49:333–344. [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider M, Schaer M, Mutlu AK, Menghetti S, Glaser B, Debbané M, et al. Clinical and cognitive risk factors for psychotic symptoms in 22q11.2 deletion syndrome: a transversal and longitudinal approach. Eur Child Adolesc Psychiatry. 2014;23:425–436. doi: 10.1007/s00787-013-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res. 2010;118:118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armando M, Girardi P, Vicari S, Menghini D, Digilio MC, Pontillo M, et al. Adolescents at ultra-high risk for psychosis with and without 22q11 deletion syndrome: a comparison of prodromal psychotic symptoms and general functioning. Schizophr Res. 2012;139:151–156. doi: 10.1016/j.schres.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monks S, Niarchou M, Davies AR, Walters JT, Williams N, Owen MJ, et al. Further evidence for high rates of schizophrenia in 22q11.2 deletion syndrome. Schizophr Res. 2014;153:231–236. doi: 10.1016/j.schres.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophr Res. 2011;129:20–28. doi: 10.1016/j.schres.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 30.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell ME. Manual for the FIGS. Clinical Neurogenetics Branch, National Institute of Mental Health; Bethesda (MD): 1992. [Google Scholar]
- 33.Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT4). Psychological Assessment Resources, Lutz. 2006 [Google Scholar]
- 34.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychiatric Association APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Publishing; Washington DC: 2000. [Google Scholar]
- 38.IBM SPSS Statistics for Windows, Version 23.0. IBM Corp.; Armonk, NY.: 2015. [Google Scholar]
- 39.Osterlind SJ, Everson HT. Differential item functioning. Sage Publications; 2009. [Google Scholar]
- 40.Addington J, Liu L, Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2): The Prodromal Symptoms. J Nerv Ment Dis. 2015;203:328–335. doi: 10.1097/NMD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kates WR, Russo N, Wood WM, Antshel KM, Faraone SV, Fremont WP. Neurocognitive and familial moderators of psychiatric risk in velocardiofacial (22q11.2 deletion) syndrome: a longitudinal study. Psychol Med. 2015;45:1629–1639. doi: 10.1017/S0033291714002724. [DOI] [PubMed] [Google Scholar]
- 43.Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 44.Mollon J, David AS, Morgan C, Frissa S, Glahn D, Pilecka I, et al. Psychotic Experiences and Neuropsychological Functioning in a Population-based Sample. JAMA Psychiatry. 2016;73:129–138. doi: 10.1001/jamapsychiatry.2015.2551. [DOI] [PubMed] [Google Scholar]
- 45.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71:366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 46.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 47.Dalsgaard S, Mortensen PB, Frydenberg M, Maibing CM, Nordentoft M, Thomsen PH. Association between Attention-Deficit Hyperactivity Disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry. 2014;29:259–263. doi: 10.1016/j.eurpsy.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Shyu YC, Yuan SS, Lee SY, Yang CJ, Yang KC, Lee TL, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: A nationwide population-based study in Taiwan. Schizophr Res. 2015;168:161–167. doi: 10.1016/j.schres.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 49.McDonald-McGinn DM, Minugh-Purvis N, Kirschner RE, Jawad A, Tonnesen MK, Catanzaro JR, et al. The 22q11.2 deletion in African-American patients: an underdiagnosed population? Am J Med Genet A. 2005;134:242–246. doi: 10.1002/ajmg.a.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.