Abstract
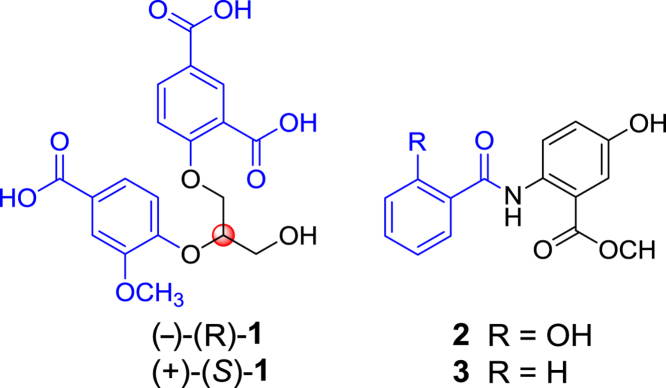
A pair of new diphenyl glycerol ether enantiomers (−)-1 and (+)-1 and two new methyl benzamidobenzoates 2 and 3, named (−)-(R)- and (+)-(S)-isatindigotrioic acid [(−)-1 and (+)-1] and isatindigoticamides A (2) and B (3), respectively, were isolated from an aqueous decoction of the roots of Isatis indigotica (ban lan gen). Their structures were elucidated by spectroscopic data analysis including 2D NMR experiments. The absolute configurations of (−)-1 and (+)-1 were assigned based on the CD exciton chirality method. Compounds 2 and 3 exhibited antiviral activities against HSV-1 with IC50 values of 4.87 and 25.87 μmol/L, respectively. Compound 2 was also found active against Coxsackie virus B3 and LPS-induced NO production.
KEY WORDS: Cruciferae, Isatis indigotica, Aromatic metabolite, Antiviral activity
Graphical abstract
A pair of unusual new diphenyl glycerol ether enantiomers (−)-(S)-1 and (+)-(R)-1 and two new methyl benzamidobenzoates 2 and 3 were isolated from Chinese traditional herbal medicine “ban lan gen” (Isatis indigotica roots). The enantiomers were separated by HPLC on a chiral column. Compound 2 showed activities against replication of HSV-1 and Coxsackie virus B3 and against the LPS-induced NO production in mouse peritoneal macrophage. Compound 3 was active against HSV-1. These results, together with previous studies, demonstrate that the diverse chemical constituents with varied activities contribute to pharmacological efficacy that supports the traditional application of this herbal medicine.
1. Introduction
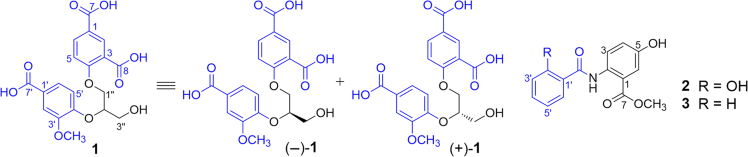
“Ban lan gen”, the dried roots of Isatis indigotica Fort. (Cruciferae), is an important traditional Chinese medicine mainly used for the treatment of influenza and infection diseases1. Previous studies showed that extracts of “ban lan gen” had diverse biological activities and contained various types of active constituents2, 3, 4, 5, 6, 7, 8, 9. However, the previous studies were mainly focused on ethanol or methanol extracts, which is not in agreement with practical application of the herbal medicine by decocting with water. Therefore, an aqueous decoction of “ban lan gen” was investigated as part of our program to systematically study the chemical and biological diversity of several Chinese traditional medicines10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. From the decoction, 43 new alkaloids and 54 known compounds, including a pair of indole alkaloid enantiomers containing dihydrothiopyran and 1,2,4-thiadiazole rings, a pair of bisindole alkaloid enantiomers, four stereoisomers of 3,5-bis(2-hydroxybut-3-en-1-yl)-1,2,4-thiadiazole, and twelve glycosidic indole alkaloids, as well as their antiviral and/or hepatocyte-protective activities, were characterized23, 24, 25, 26, 27, 28, 29, 30. Continuous investigation on remaining fractions from the decoction resulted in the separation of a pair of unusual new diphenyl glycerol ether enantiomers (−)-1 and (+)-1 and two new methyl benzamidobenzoates (2 and 3) (Fig. 1). Herein, reported are details of the isolation, structure elucidation and biological activity of these compounds.
Figure 1.
The structures of enantiomer mixture 1 and compounds (−)- and (+)-1 and 2 and 3.
2. Results and discussion
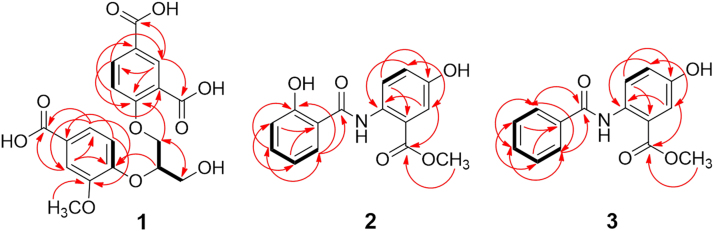
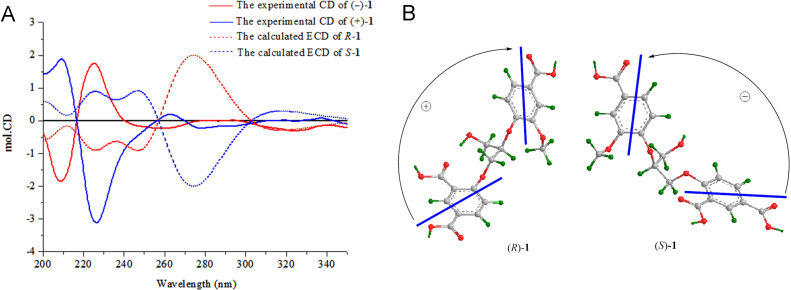
Compound 1 was isolated as a white amorphous powder with [α]20D ≈ 0 (c 0.23, MeOH). The IR spectrum of 1 showed the presence of hydroxyl (3433 cm—1), conjugated carbonyl (1705 and 1634 cm—1), and aromatic ring (1604 and 1496 cm—1) functional groups. The molecular formula of 1 was determined as C19H18O10 by HR-ESI-MS at m/z 407.0986 [M+H]+ (Calcd. for C19H19O10 407.0973) combined with the NMR data (Table 1). The 1H NMR spectrum of 1 in DMSO-d6 showed signals attributable to two 3,4-disubstituted phenyl moieties [δH 8.31 (d, J = 2.4 Hz, H-2), 6.60 (d, J = 9.0 Hz, H-5), and 7.66 (dd, J=9.0 and 2.4 Hz, H-6) and 7.48 (d, J = 1.8 Hz, H-2′), 6.97 (d, J = 8.4 Hz, H-5′), and 7.40 (brd, J=8.4 Hz, H-6′)], two oxygen-bearing methylenes [δH 4.43 (dd, J = 12.0 and 3.6 Hz, H-1ʺa), 4.33 (dd, J=6.0, 12.0 Hz, H-1ʺb), and 3.69 (brd, J=6.0 Hz, H2−3ʺ)], one oxygen-bearing methine [δH 4.50 (m, H-2ʺ)], and a methoxy group [δH 3.70 (s)]. The 13C NMR spectrum of 1 exhibited carbon signals corresponding to the above units and three additional carboxylic carbons resonated at δC 170.4 (C-8) and 169.8 and 169.7 (C-7 and C-7′), respectively. These spectroscopic data suggest that 1 is an unusual natural product consisting of two 3,4-disubstituted phenyl moieties, three carboxylic groups, and a glycerol unit. This was confirmed by 2D NMR data analysis of 1. The 1H—1H COSY cross peak between H-5 and H-6 and HMBC correlations from H-2 to C-4, C-6, C-7, and C-8; from H-5 to C-1 and C-3; from H-6 to C-2, C-4, and C-7 (Fig. 2), together with their chemical shifts, indicated the presence of a 4-substituted isophthalic acid moiety in 1. Meanwhile, the 1H—1H COSY correlation between H-5′ and H-6′ and the HMBC correlations from H-2′ to C-6′, C-4′, and C-7′; from H-5′ to C-1′ and C-3′; from H-6′ to C-4′, C-2′ and C-7′, and from OCH3 to C-4′, in combination with the chemical shifts of these proton and carbon resonances, demonstrated that there was a 4′-substituted 3′-methoxybenzoic acid moiety. The existence of glycerol unit in 1 was verified by the 1H—1H COSY correlations H2-1ʺ/H-2ʺ/H2-3ʺ and their chemical shifts, as well as by the HMBC correlations from H-1ʺ to C-2ʺ and C-3ʺ; from H-2ʺ to C-1ʺ and C-3ʺ, and from H-3ʺ to C-1ʺ and C-2ʺ. In addition, the HMBC correlations from H-1ʺ to C-4 and from H-2ʺ to C-4′, along with the molecular formula, revealed that C-1ʺ and C-2ʺ of the glycerol unit were linked via ether bonds with C-4 and C-4′ of the two aromatic acid moieties, respectively, providing the planar structure for 1. The optical inactivity of 1 indicated that it was obtained as a racemate, which was proved by HPLC analysis on an analytical chiral column displayed two peaks with around 1:1 integration ratio. Subsequent HPLC separation of 1 using a semi-preparative chiral column yielded (−)-1 {[α]20D −12.7 (c 0.06, MeOH)} and (+)-1 {[α]20D +11.8 (c 0.05, MeOH)}, which had the consistent 1H NMR spectroscopic data with those of 1 prior to separation. To determine the configurations of (−)-1 and (+)-1, the electronic circular dichroism (ECD) spectra of (−)-1 and (+)-1 were calculated using the time-dependent density functional theory (TDDFT) method31. However, the calculated ECD spectra completely differed from the experimental circular dichroism (CD) spectra (Fig. 3). This indicates that comparison of the calculated ECD and the experimental CD spectra is not applicable for determination of the absolute configuration of (−)-1 and (+)-1. An explanation is from flexibility of the structure, of which dynamic conformations under experimental conditions would not be consistent with the theoretically calculated conformers. The CD spectra of (−)-1 and (+)-1 displayed mirror curves with typical exciton-split Cotton effects at 207 and 225 nm, arising from interaction between the 1La transition moments of two benzoate chromophores32. Based on the exciton chirality method, in the CD spectrum of (−)-1, the split Cotton effects with a positive sign (a positive first Cotton effect and a negative second Cotton effect) indicated that the exciton coupling of two benzoate chromophores had positive chirality (Fig. 3). This predicts the R-configuration for (−)-1. Whereas the split Cotton effects with a negative sign in the CD spectrum of (+)-1 revealed negative chirality of the two chromophores, predicting the S-configuration. Therefore, the structures of compounds (−)-1 and (+)-1 were determined and named (−)-(R)- and (+)-(S)-isatindigotrioic acid, respectively.
Table 1.
NMR spectral data (δ)a for compounds (−)-/(+)-1, 2 and 3.
| (−)-/(+)-1b |
2c |
3d |
||||
|---|---|---|---|---|---|---|
| No. | δH | δC | δH | δC | δH | δC |
| 1 | 119.1 | 118.6 | 117.2 | |||
| 2 | 8.31 d (2.4) | 132.1 | 133.8 | 135.0 | ||
| 3 | 115.8 | 8.57 d (9.0) | 123.5 | 8.76 d (9.0) | 122.4 | |
| 4 | 165.8 | 7.18 dd (9.0, 3.0) | 122.4 | 7.16 dd (9.0, 2.5) | 122.2 | |
| 5 | 6.60 d (9.0) | 116.9 | 154.0 | 153.0 | ||
| 6 | 7.66 dd (9.0, 2.4) | 133.0 | 7.56 d (3.0) | 117.5 | 7.55 d (2.5) | 117.0 |
| 7 | 169.8e | 169.4 | 169.1 | |||
| 8 | 170.4 | |||||
| 1′ | 134.8 | 116.1 | 135.8 | |||
| 2′ | 7.48 d (1.8) | 113.5 | 162.8 | 8.01 d (7.0) | 127.6 | |
| 3′ | 148.8 | 6.97 dd (8.0, 1.0) | 119.2 | 7.58 t (7.0) | 129.4 | |
| 4′ | 147.6 | 7.50 dt (1.0, 8.0) | 135.4 | 7.62 t (7.0) | 132.3 | |
| 5′ | 6.97 d (8.4) | 115.5 | 7.02 dt (1.0, 8.0) | 120.0 | 7.58 t (7.0) | 129.4 |
| 6′ | 7.40 brd (8.4) | 121.7 | 7.83 dd (8.0, 1.0) | 127.2 | 8.01 d (7.0) | 127.6 |
| 7′ | 169.7e | 169.2 | 164.9 | |||
| 1″a | 4.43 dd (12.0, 3.6) | 62.9 | ||||
| 1″b | 4.33 dd (12.0, 6.0) | |||||
| 2″ | 4.50 m | 78.0 | ||||
| 3″ | 3.69 brd (6.0) | 60.0 | ||||
| OMe | 3.70 s | 55.3 | 3.99 s | 53.2 | 3.96 s | 52.7 |
Data were measured in DMSO-d6 for (−)-/(+)-1 (600 MHz for 1H NMR and 150 MHz for 13C NMR) and in Me2CO-d6 for 2 and 3 (500 MHz for 1H NMR and 125 MHz for 13C NMR), respectively. The assignments were based on DEPT, 1H—1H COSY, HSQC, HMQC and HMBC experiments.
Data for the hydroxyl group in (−)-/(+)-1: δH 4.98 (1H, brs, OH−3″).
Data for the hydroxyl and amino groups in 2: δH 12.22 (1H, brs, OH−5), 11.89 (1H, brs, OH−2′) and 8.65 (1H, brs, NH−2).
Data for the hydroxyl and amino groups in 3: δH 11.68 (1H, brs, OH−5) and 8.75 (1H, brs, NH−2).
Data in the same column may be exchanged.
Figure 2.
Main 1H—1H COSY (thick lines) and HMBC (arrows, from 1H to 13C) correlations of 1−3.
Figure 3.
(A) The experimental CD (full lines) and calculated ECD (dash lines) spectra of (−)-1 (blue) and (+)-1 (red). (B) Illustration of the exciton chirality method predicting the absolute configurations of (−)-1 and (+)-1.
Compound 2 was obtained as colorless needles (acetone). Its IR spectrum showed absorption bands for hydroxyl and amino (3356 and 3275 cm—1), conjugated carbonyl (1691 and 1648 cm—1), and aromatic ring (1611 and 1509 cm—1) functionalities. The molecular formula of 2 was established as C15H13NO5 by (+)-HR-ESI-MS at m/z 310.0688 [M+Na]+. The NMR spectroscopic data (Table 1) revealed the presence of ortho-meta-trisubstituted and ortho-disubstituted benzene rings, a methoxy group, two carbonyls, and three exchangeable protons in 2. This suggests that 2 is a natural product containing two benzoyl moieties with substitution of the hydroxyl, amino, and methoxy groups. Connections of the structural units in 2 were further elucidated by 2D NMR experiments. The 1H—1H COSY cross-peak between H-3 and H-4 and the HMBC correlations from H-3 to C-1 and C-5, from H-4 to C-2 and C-6, from H-6 to C-2, C-4, and C-7, and from OCH3 to C-7, along with their chemical shifts, indicated the presence of a methyl N-substituted 2-amino-5-hydroxybenzoate moiety in 2. The 1H−1H COSY spectrum also showed the vicinal coupling correlations of H-3′/H-4′/H-5′/H-6′. This, combined with the HMBC correlations from H-3′ to C-1′, C-5′, from H-4′ to C-2′ and C-6′, from H-5′ to C-1′ and C-3′, and from H-6′ to C-2′, C-4′ and C-7′, as well as with their chemical shifts, revealed the occurrence of a 2′-hydoxybenzoyl moiety in 2. Although no information was obtained for a connection of two moieties, to satisfy the requirement of the molecular formula, the two moieties must be connected via an amide bond. This was supported by comparison of the NMR data with the reported data for dianthramide B33. Therefore, compound 2 was determined as methyl 5-hydroxy-2-(2′-hydroxybenzamido)benzoate and named isatindigoticamide A.
Compound 3, colorless needles (acetone), has the molecular formula of C15H13NO4 as determined by HR-ESI-MS and NMR data. Comparison of the NMR spectroscopic data of 3 and 2 indicated that the 2′-hydroxybenzoyl moiety in 2 was replaced by a benzoyl unit in 3. This suggests 3 is the analogue of 2 without the 2′-hydroxy group, which was confirmed by 2D NMR data analysis. Especially, the 1H—1H COSY cross-peaks of H-2′(H-6′)/H-3′(H-5′)/H-4′ and the HMBC correlations from H-2′(H-6′) to C-7′ verified the presence of an unsubstituted benzoyl moiety in 3. Therefore, compound 3 was determined as methyl 2-(benzamido)-5-hydroxybenzoate and named isatindigoticamide B.
In the preliminary in vitro assays carried out in our studies, compounds 2 and 3 showed antiviral activity against herpes simplex virus1 (HSV-1)24 with IC50 values of 4.87 and 25.87 μmol/L and SI values of 8.82 and 2.68, respectively, while the positive control acyclovir gave the IC50 and SI values of 0.71 μmol/L and 140.85. Compound 2 was also active against Coxsackie virus B3 with the IC50 and SI values of 8.62 μmol/L and 4.98, respectively (the positive control pleconaril gave IC50 0.41 μmol/L and SI 243.9). In addition, compounds 2 and 3 exhibited inhibitory activity against the LPS-induced NO production in mouse peritoneal macrophage25 with inhibitions of 86.9% and 39.6% at 10 μmol/L, and the positive control dexamethasone had 92.0% inhibition at the same concentration. Comparison of the structures between 2 and 3 suggests that the presence of hydroxyl group at C-2′ may enhance the activities. Due to limitation of the sample amounts, the enantiomers (−)-/(+)-1 were not assayed.
3. Conclusions
Four new natural products, including a pair of new diphenyl glycerol ether enantiomers (−)-/(+)-1 and two new methyl benzamidobenzoates 2 and 3, were isolated and structurally determined from the aqueous decoction of Chinese traditional herbal medicine "ban lan gen". The enantiomers were separated by HPLC on a chiral semi-preparative column and their absolute configurations were determined by the CD exciton chirality method. Among the new isolates, compound 2 exhibited activities against replication of HSV-1 and Coxsackie virus B3 and against the LPS-induced NO production in mouse peritoneal macrophage. Compound 3 was only active against HSV-1. This result, together with our previous studies, continuously illustrate that the diverse chemical constituents with varied activities have contributions to pharmacological efficacy that supports the traditional usage of ban lan gen. Although the enantiomers (−)-/(+)-1 were not assayed due to limitation of the sample amounts, these compounds provide new model structures for further synthesis and biological evaluation. In particular, as compared with 3, an enhancement of activity by the C-2′ hydroxyl group in 2 provides an important clue for in-depth studies of structural modification and structure–activity relationship, as well as new drug development based on the drug-like methyl benzamidobenzoates.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured on a P-2000 polarimeter (JASCO, Tokyo, Japan). UV spectra were obtained on a V-650 spectrometer (JASCO, Tokyo, Japan). IR spectra were acquired on a Nicolet 5700 FT-IR microscope instrument (FT-IR microscope transmission) (Thermo Electron Corporation, Madison, WI, USA). NMR spectra were measured at 500 MHz or 600 MHz for 1H NMR, and 125 MHz or 150 MHz for 13C NMR, respectively, on Inova 500 or VNS 600 (Varian Associates Inc., Palo Alto, CA, USA) in DMSO-d6 or Me2CO-d6, using solvent peaks as references. ESI-MS and HR-ESI-MS data were collected on an AccuToFCS JMS-T100CS spectrometer (Agilent Technologies, Ltd., Santa Clara, CA, USA). Column chromatography (CC) was conducted on macroporous adsorbent resin (HPD-110, Cangzhou Bon Absorber Technology Co., Ltd., Cangzhou, China), silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), CHP 20P (Mitsubishi Chemical Inc., Tokyo, Japan), or reversed phase C-18 silica gel (W. R. Grace & Co., MD, USA). HPLC separation was carried out on an instrument equipped with an Agilent Chem Station for LC system, an Agilent 1200 pump, and an Agilent 1100 single-wavelength absorbance detector (Agilent Technologies, Ltd.) using a Grace semi-preparative column (250 mm×10 mm i.d.) packed with C18 reversed phase silica gel (5 μm) (W. R. Grace & Co., MD, USA) or a Chiralpak AD-H column (250 mm×10 mm i.d.) packed with amylose tris(3,5-dimethylphenylcarbamate) coated on 5 μm silica gel (Daicel Chiral Technologies Co., Ltd., Shanghai, China). TLC was carried out on glass precoated silica gel GF254 plates (Qingdao Marine Chemical Inc.). Spots were visualized under UV light or by spraying with 7% H2SO4 in 95% EtOH followed by heating. Unless otherwise noted, all chemicals were purchased from commercially available sources and were used without further purification.
4.2. Plant material
The roots of I. indigotica were collected in December 2009 from Anhui Province, China. Plant identity was verified by Mr. Lin Ma (Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China). A voucher specimen (No. ID-S-2385) was deposited at the herbarium of Natural Medicinal Chemistry, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College.
4.3. Extraction and isolation
The air-dried and pulvarized plant material (50 kg) was decocted with H2O (150 L, 3×1 h). The aqueous extracts were combined and evaporated under reduced pressure to yield a dark-brown residue (32 kg). The residue was dissolved in H2O (122 L), loaded on a macroporous adsorbent resin (HPD-110, 19 kg) column (200 cm×20 cm), and eluted successively with H2O (50 L), 50% EtOH (125 L), and 95% EtOH (100 L) to yield three corresponding fractions A, B and C. After removing the solvent under reduced pressure, fraction B (0.9 kg) was separated by CC over MCI gel CHP 20 P (5 L), with successive elution using H2O (10 L), 30% EtOH (30 L), 50% EtOH (20 L), 95% EtOH (10 L), and Me2CO (8 L), to give fractions B1–B5. Fraction B3 (165 g) was chromatographed over silica gel, eluted by a gradient of increasing MeOH (0–100%) in EtOAc to yield B3-1–B3-16. Fraction B3-4 (11.0 g) was further fractionated by CC over Sephadex LH-20 (MeOH) to yield B3-4-1−B3-4-9, of which B3-4-5 (2.5 g) was separated by reversed phase medium pressure liquid chromatography (RP-MPLC), eluted with a gradient of increasing MeOH (20%–100%) in H2O, to give B3-4-5-1–B3-4-5-31. Fraction B3-4-5-15 (136.0 mg) was re-chromatographed over Sephadex LH-20 (MeOH) to yield B3-4-5-15-1–B3-4-5-15-6. Isolation of B3-4-5-15-3 (10.0 mg) by reversed phase semi-preparative HPLC (C18 column, 40% MeOH in H2O, containing 0.3% AcOH, 1.5 mL/min) gave 1 (2.0 mg, tR=55 min). Subsequent separation of 1 by HPLC using the Chiralpak AD-H column (n-hexane-iPrOH, 4:1, containing 0.1% TFA, v/v/v, 1.5 mL/min) yielded (−)-1 (0.74 mg, tR=35.3 min) and (+)-1 (0.64 mg, tR=40.6 min). Fraction C (88.0 g) was fractionated by CC over silica gel, eluted with a gradient of increasing acetone concentration (0–100%) in petroleum ether, to yield C1–C11. Subfraction C7 (11.2 g) was subjected to CC over Sephadex LH-20 (CHCl3-MeOH, 1:1, v/v) to give C7-1–C7-6, of which C7-4 (810 mg) was separated by reversed phase flash CC, eluted with a gradient of increasing MeOH (55%−95%) in water to yield 2 (220.6 mg) and 3 (5.3 mg).
4.3.1. (−)-(R)- and (+)-(S)-isatindigotrioic acid [(−)- and (+)-1]
Mixture of (−)-1 and (+)-1 in around 1:1 ratio (1), white amorphous power; [α]20D ≈ 0.0 (c 0.23, MeOH); UV (MeOH) λmax (logε) 209 (4.28), 258 (3.82), 292 (sh, 3.43) nm; IR νmax 3433, 1705, 1634, 1604, 1562, 1496, 1384, 1278, 1218, 1188, 1117, 1049, 1027, 1002, 943, 887, 769, 703 cm−1; 1H NMR (DMSO-d6, 600 MHz) spectral data, see Table 1; 13C NMR (DMSO-d6, 150 MHz) spectral data, see Table 1; ESI-MS m/z 405 [M-H]−; HR-ESI-MS m/z 407.0986 [M+H]+ (Calcd. for C19H19O10 407.0973), 429.0806 [M+Na]+ (Calcd. for C19H18O10Na 429.0792). (−)-1: [α]20D −12.7 (c 0.06, MeOH); CD (MeOH) 208 (Δε −0.74), 225 (Δε +0.70); (+)-1: [α]20D +11.8 (c 0.05, MeOH); CD (MeOH) 207.5 (Δε +1.88), 225 (Δε −3.11).
4.3.2. Isatindigoticamide A (2)
Colorless needles, mp 156–158 °C; UV (MeOH) λmax (logε) 208 (4.63), 239 (4.30), 309 (4.10), 338 (4.09) nm; IR (KBr) νmax 3356, 3275, 1691, 1648, 1611, 1546, 1509, 1451, 1357, 1323, 1260, 1228, 1176, 1095, 1073, 1038, 984, 903, 845, 828, 786, 747, 685, 567 cm—1; 1H NMR (Me2CO-d6, 500 MHz) spectral data, see Table 1; 13C NMR (Me2CO-d6, 125 MHz) spectral data, see Table 1; ESI-MS m/z 286 [M–H]−; HR-ESI-MS m/z 310.0688 [M+Na]+ (Calcd. for C15H13NO5 310.0686).
4.3.3. Isatindigoticamide B (3)
Colorless needles, mp 171–173 °C; UV (MeOH) λmax (log ε) 203 (4.38), 221 (4.37), 281 (4.00), 339 (3.86) nm; IR (KBr) νmax 3255, 3173, 2953, 1701, 1639, 1600, 1544, 1502, 1428, 1357, 1303, 1254, 1232, 1192, 1119, 1074, 985, 928, 835, 794, 700, 587, 556 cm—1; 1H NMR (Me2CO-d6, 500 MHz) spectral data, see Table 1; 13C NMR (Me2CO-d6, 125 MHz) spectral data, see Table 1; ESI-MS m/z 294 [M+Na]+, 565 [2 M+Na]+, 270 [M-H]−, 541 [2M-H]−; HR-ESI-MS m/z 294.0733 [M+Na]+ (Calcd. for C15H13NO4Na 294.0737).
Acknowledgments
Financial support from the National Natural Sciences Foundation of China (NNSFC; Grant Nos. 81373287, 30825044 and 21132009), the Beijing Excellent Talent Training Project (Grant No. 2013D009008000002), and the National Science and Technology Project of China (Nos. 2012ZX09301002-002 and 2011ZX0 9307-002-01) is acknowledged. We thank Chinese Academy of Medical Sciences and Peking Union Medical College High Performance Computing Platform for supporting the calculation of the ECD spectra.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at 10.1016/j.apsb.2016.09.004.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Jiangsu New Medical College . Vol. 1. Shanghai Science and Technology Publishing House; Shanghai: 1986. (Dictionary of Traditional Chinese Medicine.). . p. 126, 1250. [Google Scholar]
- 2.Ho Y.L., Chang Y.S. Studies on the antinociceptive, anti-inflammatory and antipyretic effects of Isatis indigotica root. Phytomedicine. 2002;9:419–424. doi: 10.1078/09447110260571661. [DOI] [PubMed] [Google Scholar]
- 3.Fang J.G., Liu Y.H., Wang W.Q., Xie W., Fang S.X., Han H.G. The anti-endotoxic effect of o-aminobenzoic acid from Radix Isatidis. Acta Pharm Sin. 2005;26:593–597. doi: 10.1111/j.1745-7254.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 4.Hsuan S.L., Chang S.C., Wang S.Y., Liao T.L., Jong T.T., Chien M.S. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol. 2009;123:61–67. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo L., Li J.B., Xu J., Yang J.Z., Zhang D.M., Tong Y.L. Studies on chemical constituents in root of Isatis indigotica. Chin J Chin Mater Med. 2007;32:688–691. [PubMed] [Google Scholar]
- 6.Sun D.D., Dong W.W., Li X., Zhang H.Q. Isolation, structural determination and cytotoxic activity of two new ceramides from the root of Isatis indigotica. Sci China Ser B Chem. 2009;52:621–625. [Google Scholar]
- 7.Wu Y., Zhang Z.X., Hu H., Li D., Qiu G., Hu X. Novel indole C-glycosides from Isatis indigotica and their potential cytotoxic activity. Fitoterapia. 2011;82:288–292. doi: 10.1016/j.fitote.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z., Shi Y., Wang Z., Wang R., Li Y. Biotransformation of glucosinolates epiprogoitrin and progoitrin to (R)- and (S)-goitrin in Radix isatidis. J Agric Food Chem. 2011;59:12467–12472. doi: 10.1021/jf203321u. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Wang G., Wang M., Jiang H., Chen L., Zhao F. Indole alkaloid from the roots of Isatis indigotica and their inhibitory effects on nitric oxide production. Fitoterapia. 2014;95 doi: 10.1016/j.fitote.2014.03.019. 175. [DOI] [PubMed] [Google Scholar]
- 10.Zhao F., Wang S.J., Lin S., Zhu C.G., Yue Z.G., Yu Y. Natural and unnatural anthraquinones isolated from the ethanol extract of the roots of Knoxia valerianoides. Acta Pharm Sin B. 2012;2:260–266. [Google Scholar]
- 11.Yu Y., Zhu C.G., Wang S.J., Song W.X., Yang Y.C., Shi J.G. Homosecoiridoid alkaloids with amino acid units from the flower buds of Lonicera japonica. J Nat Prod. 2013;76:2226–2233. doi: 10.1021/np4005773. [DOI] [PubMed] [Google Scholar]
- 12.Wang F., Jiang Y.P., Wang X.L., Wang S.J., Bu P.B., Lin S. Aromatic glycosides from the flower buds of Lonicera japonica. J Asian Nat Prod Res. 2013;15:492–501. doi: 10.1080/10286020.2013.785531. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z.B., Jiang B.Y., Zhu C.G., Guo Q.L., Peng Y., Wang X.L. Aromatic acid derivatives from the lateral roots of Aconitum carmichaelii. J Asian Nat Prod Res. 2014;16:891–900. doi: 10.1080/10286020.2014.939585. [DOI] [PubMed] [Google Scholar]
- 14.Tian Y., Guo Q.L., Xu W.D., Zhu C.G., Yang Y.C., Shi J.G. A minor diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina. Org Lett. 2014;16:3950–3953. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 15.Song W.X., Yang Y.C., Shi J.G. Two new β-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica: isolation, structure elucidation, semisynthesis, and biological activities. Chin Chem Lett. 2014;25:1215–1219. [Google Scholar]
- 16.Xu W.D., Tian Y., Guo Q.L., Yang Y.C., Shi J.G. Secoeuphoractin, a minor diterpenoid with a new skeleton from Euphorbia micractina. Chin Chem Lett. 2014;25:1531–1534. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z.B., Song W.X., Shi J.G. Two 1-(6′-O-acyl-β-d-glucopyrayl)pyridinium-3-carboxylates from the flower buds of Lonicera japonica. Chin Chem Lett. 2015;26:69–72. [Google Scholar]
- 18.Yu Y., Jiang Z., Song W., Yang Y., Li Y., Jiang J. Glucosylated caffeoylquinic acid derivatives from the flower buds of Lonicera japonica. Acta Pharm Sin B. 2015;5:210–214. doi: 10.1016/j.apsb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Q.L., Wang Y.N., Zhu C.G., Chen M.H., Jiang Z.B., Chen N.H. 4-Hydroxybenzyl-substituted glutathione derivatives from Gastrodia elata. J Asian Nat Prod Res. 2015;17:439–454. doi: 10.1080/10286020.2015.1040000. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y., Liu Y., Guo Q., Jiang Z., Xu C., Zhu C. Acetylenes and fatty acids from Codonopsis pilosula. Acta Pharm Sin B. 2015;5:215–222. doi: 10.1016/j.apsb.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y.P., Liu Y., Guo Q., Jiang Z.B., Xu C.B., Zhu C.G. C14-polyacetylene glucosides from Codonopsis pilosula. J Asian Nat Prod Res. 2015;17:601–614. doi: 10.1080/10286020.2015.1041932. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q.L., Wang Y.N., Lin S., Zhu C.G., Chen M.H., Jiang Z.B. 4-Hydroxybenzyl-substituted amino acid derivatives from Gastrodia elata. Acta Pharm Sin B. 2015;5:350–357. doi: 10.1016/j.apsb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., Gan L., Lin S., Wang X., Li L., Li Y. Alkaloids from the root of Isatis indigotica. J Nat Prod. 2012;75:1167–1176. doi: 10.1021/np3002833. [DOI] [PubMed] [Google Scholar]
- 24.Chen M.H., Lin S., Li L., Zhu C.G., Wang X.L., Wang Y.N. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org Lett. 2012;14:5668–5671. doi: 10.1021/ol302660t. [DOI] [PubMed] [Google Scholar]
- 25.Wang X.L., Chen M.H., Wang F., Pu P.B., Lin S., Zhu C.G. Chemical constituents from root of Isatis indigotica. Chin J Chin Mater Med. 2013;38:1172–1182. [PubMed] [Google Scholar]
- 26.Liu Y.F., Chen M.H., Guo Q.L., Lin S., Xu C.B., Jiang Y.P. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J Asian Nat Prod Res. 2015;17:689–704. doi: 10.1080/10286020.2015.1055729. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.F., Chen M.H., Wang X.L., Zhu C.G., Lin S., Xu C.B. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin Chem Lett. 2015;26:931–936. [Google Scholar]
- 28.Liu Y.F., Chen M.H., Lin S., Li Y.H., Zhang D., Jiang J.D. Indole alkaloid glycosides from the roots of Isatis indigotica. J Asian Nat Prod Res. 2016;18:1–12. doi: 10.1080/10286020.2015.1117452. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y.F., Wang X.L., Chen M.H., Lin S., Li L., Shi J.G. Three pairs of alkaloid enantiomers from the roots of Isatis indigotica. Acta Pharm Sin B. 2016;6:141–147. doi: 10.1016/j.apsb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M.H., Lin S., Wang Y.N., Zhu C.G., Li Y.H., Jiang J.D. Antiviral stereoisomers of 3,5-bis(2-hydroxybut-3-en-1-yl)-1,2,4-thiadiazole from the roots of Isatis indigotica. Chin Chem Lett. 2016;27:643–648. [Google Scholar]
- 31.Li X.C., Ferreira D., Ding Y.Q. Determination of absolute configuration of natural products: theoretical calculation of electronic circular dichroism as a tool. Curr Org Chem. 2010;14:1678–1697. doi: 10.2174/138527210792927717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakanishi K, Berova N. Circular dichroism principles and applications. Nakanishi K, Berova N, Woody RW, editors. Wiley-VCH: New York, USA. 1994. p. 361–98
- 33.Ponchet M., Martin-Tanguy J., Marais A., Ponchet A. Dianthramides A and B, two N-benzoylanthranilic acid derivatives from elicited tissues of Dianthus caryophyllus. Phytochemistry. 1984;23:1901–1903. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material