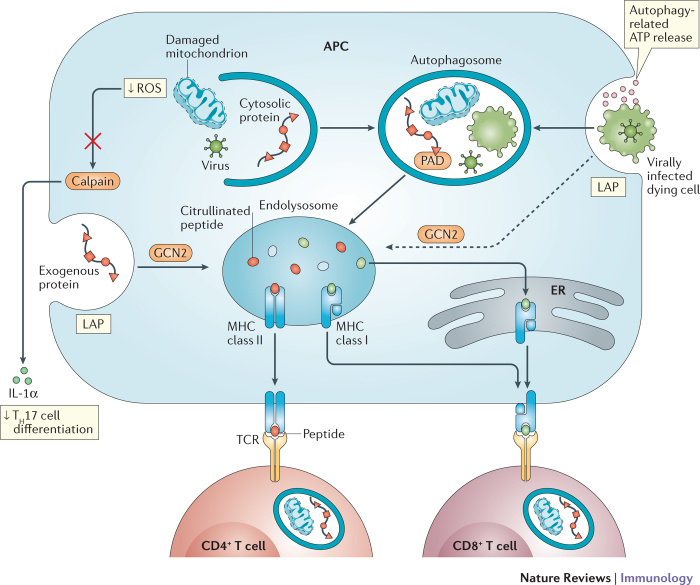
Figure 3. Autophagy coordinates a multicellular adaptive immune response.
Autophagy activity in T cells, antigen-presenting cells (APCs) and dying cells affects T cell immunity. Autophagy in APCs such as dendritic cells delivers intracellular and extracellular antigens to endolysosomal vesicles where they are loaded onto MHC class II molecules for presentation to CD4+ T cells. Peptidylarginine deiminases (PADs) in autophagosomes generate citrullinated peptides, thereby affecting the repertoire of antigens being presented. Extracellular antigens can also be delivered to the endosolysosome by LC3-associated phagocytosis (LAP). Autophagy-mediated release of ATP by dying cells, such as those infected by viruses, leads to engulfment by the APC. Internalized antigens from the dying cell can then be cross-presented on MHC class I molecules (in the endolysosome) to stimulate CD8+ T cells, a process mediated by autophagy or, potentially, LAP downstream of GCN2 activation. By inhibiting the release of key inflammatory cytokines, autophagy decreases the differentiation of CD4+ T cells into T helper 17 (TH17) cells. Alternatively, exogenous antigens may be exported into the cytosol and delivered to MHC class I molecules in the endoplasmic reticulum (ER). For instance, the removal of reactive oxygen species (ROS)-producing mitochondria inhibits calpain-mediated processing of interleukin-1α (IL-1α). Naive CD4+ T cells require autophagy for survival and proliferation (and cytokine production once activated). The establishment of memory by CD8+ T cells is also dependent on autophagy.