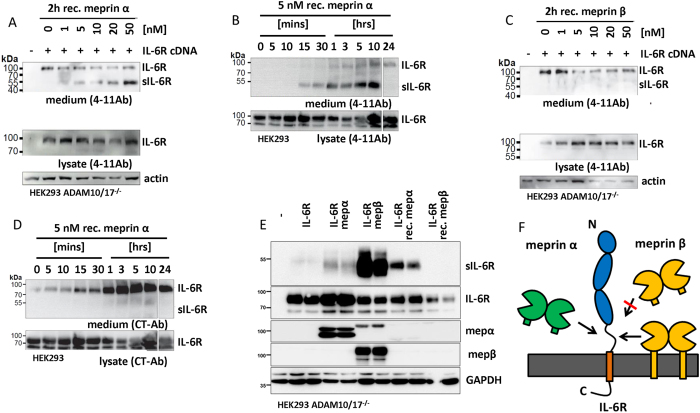
Figure 2. IL-6R cleavage by meprins generates soluble receptor independent of ADAM10/17.
(A) Human IL-6R was overexpressed in ADAM10/17 dKO HEK cells. Forty-eight hours post transfection recombinant meprin α was applied to the cell culture. After 2 hours the supernatant was harvested and ConA precipitated. A clear increase in cleaved soluble IL-6R fragment of about 50 kDa was detected with increasing concentration of meprin α. Additionally, the full-length receptor was detected in the supernatant. (B) The transfected IL-6R was also cleaved by a rather low concentration of soluble meprin α (5 nM) in a time dependent manner. Again a probable full-length version of the IL-6R was detected. (C) For soluble meprin β no cleavage product of the IL-6R was detected in the cell supernatant. However, a signal for the IL-6R with a molecular weight of the full-length receptor was observed. (D) To further address secretion of the full-length IL-6R a C-terminal antibody (CT-AB) was used, detecting the cytoplasmic part. Here, the full-length version of the IL-6R was clearly detected in cell culture supernatants at approximately 100 kDA but not the cleavage product generated by meprin α. (E) Co-transfection of the IL-6R with meprin α or meprin β revealed cleavage products generated by both proteases. As a control, recombinant meprins were added. Only meprin α and not soluble meprin β produces sIL-6R. (F) Summarizing model indicating that only soluble meprin α membrane bound meprin β shed the IL-6R. Full-length blots are shown in Supplementary Information.