Table 1. Assumptions and conclusions of significant recent works modelling carbon fluxes in single-celled phytoplankton.
| Study | Model set-up and assumptions | Conclusions |
|---|---|---|
| Holtz et al.47,48 (E. huxleyi) | 4 compartments (PY=pyrenoid, CP=chloroplast, CV=coccolith vesicle, CY=cytosol) |
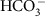 is used at low [CO2] is used at low [CO2] |
Carbonate chemistry consists of 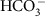 , , 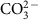 , CO2 and H+. , CO2 and H+. |
Intracellular pH gradients allow concentration of CO2 around RuBisCO without up-gradient movement of carbon. | |
Hypothesized Ca2+/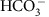 CY-to-CV transporter coupled to an H+-ATPase, with no leakage of CY-to-CV transporter coupled to an H+-ATPase, with no leakage of 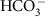 from CV from CV |
pHs: PY=5.0, CY=7.0, CP=8.0, CV=8.3–8.6 | |
Hypothesized upregulation of 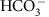 down-gradient flux into cell with decreased [CO2] in PY. down-gradient flux into cell with decreased [CO2] in PY. |
A net efflux of CO2 is not necessary to remove δ13C from cell | |
Passive CO2 and 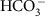 fluxes. No fluxes. No 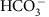 flux from CP to PY. flux from CP to PY. |
||
| CA assumed in CP and PY but not in CY and CV | ||
| Isotope model consists of 2 compartments (CY and CP) and does not consider isotopes of calcite. | ||
Membrane permeabilities to CO2 and 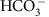 assumed, but highly heterogeneous; different for all compartments. assumed, but highly heterogeneous; different for all compartments. |
||
| Bolton & Stoll,2 (Coccolithophores) | 3 compartments (CP, CV and CY) |
 active transport to CP increases at low [CO2], at the expense of active transport to CP increases at low [CO2], at the expense of 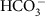 transport to CV transport to CV |
Carbonate chemistry consists of 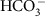 and CO2. and CO2. |
This effect is greatest in large cells. | |
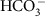 fluxes are all active and independent of [ fluxes are all active and independent of [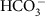 ] ] |
Difference in vital effects (δ13C calcite—δ13Cmedium) between small and large cells greatest at low [CO2] | |
| Passive CO2 fluxes. | ||
| Membrane permeabilities and CA activities assumed from Hopkinson et al.46. | ||
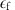 assumed (−27‰). assumed (−27‰). |
||
| Hopkinson et al.46 (Diatoms) | 1, 2 & 3 compartments (PY, CP, CY) | Membranes are highly permeable to CO2 (1.5 × 10−4–5.6 × 10−4 m s−1) |
Carbonate chemistry consists of 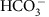 and CO2. and CO2. |
Membranes are highly impermeable to 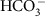 (2.5 × 10−8–2.9 × 10−7 m s−1) (2.5 × 10−8–2.9 × 10−7 m s−1) |
|
| Used 18O labelled DIC to track temporal evolution of carbonate system. | δ13C org is a function of passive diffusion of CO2, active movement of 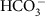 , kinetic fractionation factors associated with CA-catalysed hydration and dehydration, and the kinetic isotopic fractionation associated with RuBisCO. , kinetic fractionation factors associated with CA-catalysed hydration and dehydration, and the kinetic isotopic fractionation associated with RuBisCO. |
|
| Passive CO2 fluxes. | ||
Passive and active 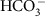 fluxes. fluxes. |
||
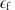 assumed (−29‰). assumed (−29‰). |
||
| Schulz et al.,26 (E. huxleyi) | 2 compartments (CP and CY) | Carbon concentrating mechanism relies upon active (ATP driven) uptake of CO2 and 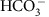
|
Carbonate chemistry consists of 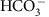 and CO2. and CO2. |
Reduction in 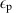 with increased with increased 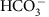 uptake into CP uptake into CP |
|
Active uptake of 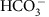 and CO2 independent of concentration and CO2 independent of concentration |
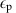 is larger when there is a greater degree of intracellular carbon leakage from the chloroplast. is larger when there is a greater degree of intracellular carbon leakage from the chloroplast. |
|
| Passive CO2 fluxes (membrane permeability to CO2=1.8 × 10−5 from76- green algae) | ||
| No efflux of HCO3 − | ||
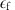 assumed (−29‰). assumed (−29‰). |
||
| Cassar et al.,25 (Diatom—Phaeodactylum tricornutum) | 2 compartments |
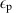 is not a unique function of is not a unique function of 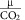
|
| Active and diffusive uptake of CO2 |
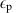 depends on leakiness of CP depends on leakiness of CP |
|
No 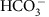 uptake uptake |
||
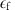 assumed (−27‰). assumed (−27‰). |
||
| Inferred fluxes based on an energy minimization approach. | ||
| Keller & Morel,24 (General phytoplankton) | 1 compartment | Downward curvature of 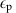 against against 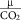 is consistent with active is consistent with active 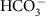 uptake contrary to32 uptake contrary to32
|
No 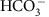 diffusion or efflux diffusion or efflux |
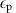 is a poor indicator of carbon substrate is a poor indicator of carbon substrate |
|
Active 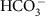 uptake scales with growth rate uptake scales with growth rate |
||
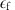 inferred from model. inferred from model. |
||
For earlier work and the evolving appreciation of the importance of cell size, shape and growth rate see Laws et al.15 and references therein.
